
- 904 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Handbook of Industrial Membranes
About this book
This manual contains necessary and useful information and data in an easily accessible format relating to the use of membranes. Membranes are among the most important engineering components in use today, and each year more and more effective uses for membrane technologies are found - for example: water purification, industrial effluent treatment, solvent dehydration by per-vaporation, recovery of volatile organic compounds, protein recovery, bioseparations and many others.The pace of change in the membrane industry has been accelerating rapidly in recent years, occasioned in part by the demand of end-users, but also as a result of the investment in R&D by manufacturers. To reflect these changes the author has obtained the latest information from some of the leading suppliers in the business. In one complete volume this unique handbook gives practical guidance to using selected membrane processes in individual industries while also providing a useful guide to equipment selection and usage.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Handbook of Industrial Membranes by K. Scott in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Chemical & Biochemical Engineering. We have over one million books available in our catalogue for you to explore.
Information
Edition
2INTRODUCTION TO MEMBRANE SEPARATIONS
SECTION 1.1 – INTRODUCTION
Membranes can be used to satisfy many of the separation requirements in the process industries. These separations can be put into two general areas; where materials are present as a number of phases and those where species are dissolved in a single phase.
A membrane is a permeable or semi-permeable phase, polymer, inorganic or metal, which restricts the motion of certain species. This membrane, or barrier, controls the relative rates of transport of various species through itself and thus, as with all separations, gives one product depleted in certain components and a second product concentrated in these components. The performance of a membrane is defined in terms of two simple factors, flux and retention or selectivity. Flux or permeation rate is the volumetric (mass or molar) flowrate of fluid passing through the membrane per unit area of membrane per unit time. Selectivity is a measure of the relative permeation rates of different components through the membrane. Retention is the fraction of solute in the feed retained by the membrane. Ideally a membrane with a high selectivity or retention and with a high flux or permeability is required, although typically attempts to maximise one factor are compromised by a reduction in the other.
Membranes are used for various separations; the separation of mixtures of gases and vapours, miscible liquids (organic mixtures and aqueous/organic mixtures) and solid/liquid and liquid/liquid dispersions and dissolved solids and solutes from liquids. The main uses of membrane separations in industry are in the:
i. The filtration of micron and submicron size particulates from liquid and gases (MF).
ii. The removal of macromolecules and colloids from liquids containing ionic species (UF).
iii. The separation of mixtures of miscible liquids (PV).
iv. The selective separation of mixtures of gases and vapour and gas mixtures (GP and VP).
v. The selective transport of only ionic species (ED).
vi. The virtual complete removal of all material, suspended and dissolved, from water or other solvents (RO).
The main feature which distinguishes membrane separations form other separation techniques is the use of another phase, the membrane. This phase, either solid, liquid or gaseous, introduces an interface(s) between the two bulk phases involved in the separation and can give advantages of efficiency and selectivity. The membrane can be neutral or charged and porous or non-porous and acts as a permselective barrier.
Transport of selected species through the membrane is achieved by applying a driving force across the membrane. This gives a broad classification of membrane separations in the way, or mechanism, material is transported across a membrane. The flow of material across a membrane is kinetically driven, by the application of either mechanical, chemical, electrical or thermal work. The important membrane processes, together with the general classification of membranes used are listed in Table 1.
TABLE 1
Membrane separations and materials.
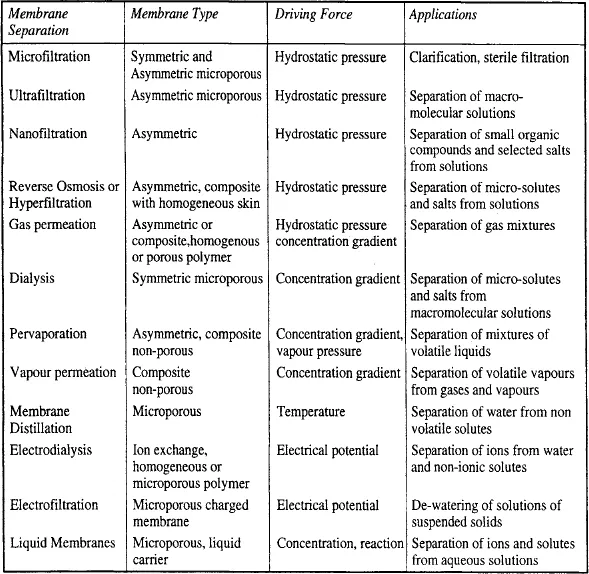
The driving force is either pressure, concentration, temperature or electrical potential. The use of driving force is not a satisfactory means of classification because apparently different membrane processes can be applied for the same separation, for example electrodialysis, reverse osmosis and pervaporation in the desalination of water. From the view of applications, classification in terms of suspended solids, colloids or dissolved solutes, etc is preferred (see Fig 1). Thus the techniques of microfiltration, ultrafiltration, employed in the category of suspended solid separation. All these processes use membranes which are microporous in nature. These are the most simplest form of membrane regarding mode of separation and consist of a solid matrix with defined pores ranging from 100 nm to 50 micron in size.
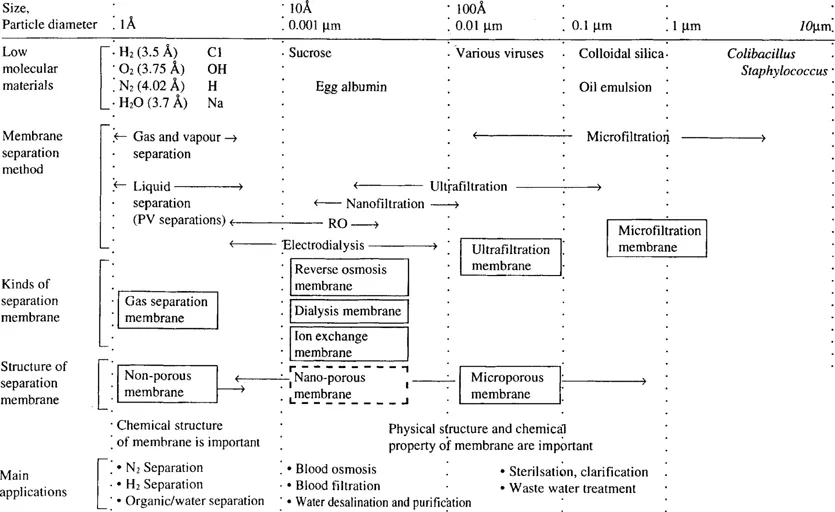
FIGURE 1 An overview of membrane separation technology.
Microfiltration (MF), in combination with ultrafiltration (UF), can solve almost any separation problem involving particulate material and macromolecules. Major technical advantages of these filtrations are that they are well suited to temperature sensitive materials and are not chemically altered as in competitive procedures such as precipitation and distillation. Membrane filtrations offer relative simplicity of operation and low costs in comparison to competition such as centrifugal separation, vacuum filtration and spray drying. The market areas for ultrafiltration are in the food and dairy industries, biotechnology, water purification and effluent treatment. The latter of these is a developing market for membrane separations as a whole. The largest market share of membrane separations is held by microfiltration and is used for clarification and sterile filtration in a wide range of industries including food and biochemical. Typical systems consist of cartridges where membranes offer absolute filtration capabilities.
A second classification of membranes under a heading homogeneous films encompass the separations; gas permeation, pervaporation, vapour permeation, reverse osmosis and nanofiltration. Separation in these cases is related directly to the transport rate of species in the membrane, determined by their diffusivity and concentration in the membrane phase. These membranes are often in the form of composites of a homogeneous film on a microporous support as used in hyperfiltration and pervaporation. The last two processes are used for similar separations, the removal of water and the concentration of solutions of ionic or organic solutes.
The membrane separations of reverse osmosis (or hyperfiltration) is not restricted to aqueous based solutions, but can in principle be applied to organic based solutions. Hyperfiltration is used in the same industries as microfiltration and ultrafiltration although a major application is in desalination to product potable water. The operating pressures of reverse osmosis are an order of magnitude grater than those of ultrafiltration and microfiltration ie 10 – 100 bar. Competition is with separations such as evaporation and distillation, where membranes score heavily because they do not involve a change in phase and do not expend energy in the latent heat of evaporation. The operating costs of membrane separations are therefore often much lower than competitive separations.
Gas permeation uses homogeneous membranes which separate species in terms of diffusivity and concentration in the membrane. This membrane technology has only recently been applied commerciall...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Preface
- Acknowledgements
- Recommended Reading
- Related Titles Published by Elsevier Science
- Chapter 1: INTRODUCTION TO MEMBRANE SEPARATIONS
- Chapter 2: MEMBRANE MATERIALS, PREPARATION AND CHARACTERISATION
- Chapter 3: GAS SEPARATIONS
- Chapter 4: AIR AND GAS FILTRATION AND CLEANING
- Chapter 5: SEPARATION OF LIQUID MIXTURES/PERVAPORATION
- Chapter 6: SEPARATION OF ORGANIC VAPOUR/AIR MIXTURES
- Chapter 7: MICROFILTRATION
- Chapter 8: ANALYTICAL APPLICATION OF MEMBRANES
- Chapter 9: WATER DESALINATION
- Chapter 10: WATER PURIFICATION
- Chapter 11: INDUSTRIAL WASTE WATER AND EFFLUENT TREATMENT
- Chapter 12: ABSORPTION, DESORPTION AND EXTRACTION WITH MEMBRANES
- Chapter 13: WASTE WATER TREATMENT AND LIQUID MEMBRANES
- Chapter 14: BIOTECHNOLOGY AND MEDICAL APPLICATIONS
- Chapter 15: MEDICAL APPLICATIONS
- Chapter 16: RECOVERY OF SALTS, ACIDS AND BASES
- Chapter 17: FOOD INDUSTRY
- Chapter 18: MEMBRANES FOR ELECTROCHEMICAL CELLS
- Chapter 19: ELECTROKINETIC SEPARATIONS
- APPENDIX
- ADVERTISERS INDEX WITH ADDRESSES, TELEPHONE AND FACSIMILE NUMBERS
- CLASSIFIED INDEX OF ADVERTISERS BY PRODUCT CATEGORY
- TRADE NAMES INDEX
- EDITORIAL INDEX