
Bioactive Food as Dietary Interventions for Diabetes
Bioactive Foods in Chronic Disease States
- 658 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Bioactive Food as Dietary Interventions for Diabetes
Bioactive Foods in Chronic Disease States
About this book
The role of diet in the prevention, control and treatment of diabetes continues to provide significant opportunity for non-pharmaceutical interventions for many of the over 20 million people who live with this disease. Looking beyond traditional dietary controls may lead to more effective, cost efficient, and flexible options for many patients.Bioactive Food as Dietary Interventions for Diabetes is the only available scientific resource focused on exploring the latest advances in bioactive food research, and the potential benefit of bioactive food choice on the diabetic condition. Written by experts from around the world, it presents important information that can help improve the health of those at risk for diabetes and diabetes related conditions using food selection as its foundation.- Focuses on the role of bioactive foods in addressing pre-diabetes symptoms, their potential to complement other treatments for those suffering from diabetes and diabetic-related obesity and other health issues- Documents foods that can affect metabolic syndrome and ways the associated information could be used to understand other diseases that share common etiological pathways- Includes insights from experts from around the world, providing global perspectives and options based on various regional foods
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Chapter 1
Role of Oxidative Stress in the Pathogenesis of Insulin Resistance and Type 2 Diabetes
Abbreviations
1 Introduction
2 Systemic Glucose Homeostasis is a Multiorgan Process
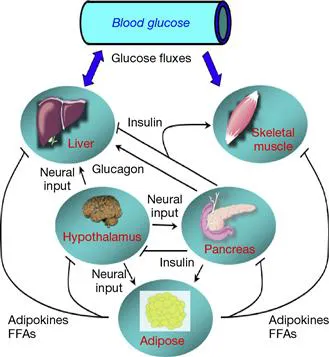
3 Glucose Dysregulation: The Pathogenesis of Insulin Resistance
4 Origins of Oxidative Stress in Various Cell Types
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Preface: Diabetes Food
- Contributors
- Chapter 1. Role of Oxidative Stress in the Pathogenesis of Insulin Resistance and Type 2 Diabetes
- Chapter 2. Diabetes and the Role of Dietary Supplements
- Chapter 3. Government Regulation of Dietary Supplements and Foods: Role in Diabetes
- Chapter 4. Diabetes as an Immune Dysfunction Syndrome
- Chapter 5. Antihyperglycemic Potential of Secoisolaricinol Diglucoside
- Chapter 6. Antidiabetic Potential of Trigonelline and 4-Hydroxyisoleucine in Fenugreek
- Chapter 7. Community Participation and Diabetes Control
- Chapter 8. Glycine max (Soybean) Treatment for Diabetes
- Chapter 9. Amino Acid Supplements and Diabetes
- Chapter 10. Reduction in Serum Glucose with Garlic Extracts
- Chapter 11. Dietary Supplements, Immune Modulation, and Diabetes Control
- Chapter 12. Dietary Supplements and Herbs in Diabetes and Its Prevention
- Chapter 13. Phytotherapeutics in Treating Diabetes
- Chapter 14. Plant-Derived Hydroxycinnamate Derivatives, Insulin Sensitivity, and Adiponectin: Implications for Diabetes Control
- Chapter 15. Antidiabetic Activity of Allium Sativum
- Chapter 16. Chromium and Diabetes
- Chapter 17. Dietary Calcium and Magnesium and the Risk of Type 2 Diabetes
- Chapter 18. Polyunsaturated Fatty Acids and Insulin Resistance
- Chapter 19. Vitamin D and Type 2 Diabetes Mellitus
- Chapter 20. Pongamia pinnata: Treatment of Diabetes
- Chapter 21. Oyster Mushroom (Pleurotus pulmonarius) and Diabetes Care
- Chapter 22. Traditional Medicinal Plants of Indigenous Peoples of Canada and Their Antioxidant Activity in Relation to Treatment of Diabetes
- Chapter 23. Indian Medicinal Plants with Hypoglycemic Potential
- Chapter 24. Plant Extracts and Alkaloids: Prevention of Diabetic Nephropathy
- Chapter 25. Lutein and Diabetic Cataracts
- Chapter 26. Compounds in Vegetables Including Okra and Fenugreek of Potential Value in the Treatment of Diabetes
- Chapter 27. Probiotics and Diabetes/Obesity: Health Implications
- Chapter 28. Tradition and Perspectives of Diabetes Treatment in Greco-Arab and Islamic Medicine
- Chapter 29. State of the Art of Diabetes Treatment in Greco-Arab and Islamic Medicine
- Chapter 30. Phytonutrients in Diabetes Management
- Chapter 31. Antidiabetic Effects of Punica granatum L (Pomegranate): A Review
- Chapter 32. Type II Diabetes Mellitus: 2011 Research Summary
- Chapter 33. Diabetes and Natural Products
- Chapter 34. L-Carnitine in Patients with Diabetes
- Chapter 35. Antioxidants and Inflammation in Obesity
- Chapter 36. Magnesium and Metabolic Syndrome: The Role of Magnesium in Health and Disease
- Chapter 37. Obesity in Ayurveda: Dietary, Lifestyle, and Herbal Considerations
- Chapter 38. The Effects of a Fermented Soy Product and Isoflavones in Metabolic Syndrome Control
- Chapter 39. Anti-Inflammatory Actions of Pycnogenol: Diabetes and Arthritis
- Chapter 40. Metabolic Syndrome: Diet, Obesity, and Chronic Inflammation
- Chapter 41. The Indian Medicinal Plant Aegle marmelos in the Treatment of Diabetes Mellitus: Promise and Prospects
- Chapter 42. Antidiabetic and Hypoglycemic Effects of Syzygium cumini (Black Plum)
- Chapter 43. Human Milk as a Bioactive Food
- Chapter 44. Ginger (Zingiber officinale Roscoe) in the Treatment of Diabetes and Metabolic Syndrome: Preclinical Observations
- Chapter 45. Antidiabetic and Cardioprotective Effects of Amla (Emblica officinalis Gaertn) and its Phytochemicals: Preclinical Observations
- Chapter 46. Prickly Pear Cactus (‘Nopal’) for the Treatment of Type 2 Diabetes Mellitus
- Chapter 47. Antioxidant Capacity of Honey: Potential Health Benefit
- Index