
Clinical Chemistry, Immunology and Laboratory Quality Control
A Comprehensive Review for Board Preparation, Certification and Clinical Practice
- 504 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Clinical Chemistry, Immunology and Laboratory Quality Control
A Comprehensive Review for Board Preparation, Certification and Clinical Practice
About this book
All pathology residents must have a good command of clinical chemistry, toxicology, immunology, and laboratory statistics to be successful pathologists, as well as to pass the American Board of Pathology examination. Clinical chemistry, however, is a topic in which many senior medical students and pathology residents face challenges. Clinical Chemistry, Immunology and Laboratory Quality Control meets this challenge head on with a clear and easy-to-read presentation of core topics and detailed case studies that illustrate the application of clinical chemistry knowledge to everyday patient care.This basic primer offers practical examples of how things function in the pathology clinic as well as useful lists, sample questions, and a bullet-point format ideal for quick pre-Board review. While larger textbooks in clinical chemistry provide highly detailed information regarding instrumentation and statistics, this may be too much information for students, residents, and clinicians. This book is designed to educate senior medical students, residents, and fellows, and to "refresh" the knowledge base of practicing clinicians on how tests are performed in their laboratories (i.e., method principles, interferences, and limitations).- Takes a practical and easy-to-read approach to understanding clinical chemistry and toxicology- Covers all important clinical information found in larger textbooks in a more succinct and easy-to-understand manner- Covers essential concepts in instrumentation and statistics in such a way that fellows and clinicians understand the methods without having to become specialists in the field- Includes chapters on drug-herb interaction and pharmacogenomics, topics not covered by textbooks in the field of clinical chemistry or laboratory medicine
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Instrumentation and Analytical Methods
Keywords
1.1 Introduction
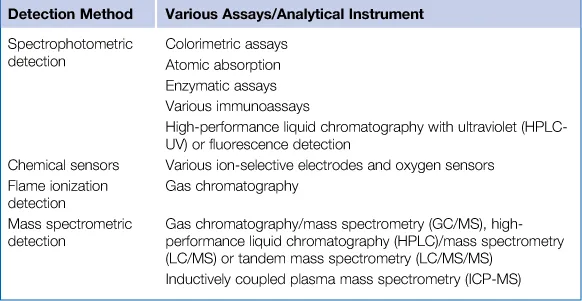
1.2 Spectrophotometry and Related Techniques
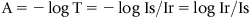
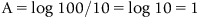
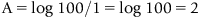
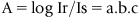
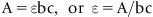
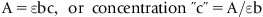
1.3 Atomic Absorption




Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Dedication
- Preface
- Chapter 1. Instrumentation and Analytical Methods
- Chapter 2. Immunoassay Platform and Designs
- Chapter 3. Pre-Analytical Variables
- Chapter 4. Laboratory Statistics and Quality Control
- Chapter 5. Water, Homeostasis, Electrolytes, and Acid–Base Balance
- Chapter 6. Lipid Metabolism and Disorders
- Chapter 7. Carbohydrate Metabolism, Diabetes, and Hypoglycemia
- Chapter 8. Cardiac Markers
- Chapter 9. Endocrinology
- Chapter 10. Liver Diseases and Liver Function Tests
- Chapter 11. Renal Function Tests
- Chapter 12. Inborn Errors of Metabolism
- Chapter 13. Tumor Markers
- Chapter 14. Therapeutic Drug Monitoring
- Chapter 15. Interferences in Therapeutic Drug Monitoring
- Chapter 16. Drugs of Abuse Testing
- Chapter 17. Challenges in Drugs of Abuse Testing: Magic Mushrooms, Peyote Cactus, and Designer Drugs
- Chapter 18. Testing for Ethyl Alcohol (Alcohol) and Other Volatiles
- Chapter 19. Common Poisonings Including Heavy Metal Poisoning
- Chapter 20. Pharmacogenomics
- Chapter 21. Hemoglobinopathy
- Chapter 22. Protein Electrophoresis and Immunofixation
- Chapter 23. Human Immunodeficiency Virus (HIV) and Hepatitis Testing
- Chapter 24. Autoimmunity, Complement, and Immunodeficiency
- Chapter 25. Effect of Herbal Supplements on Clinical Laboratory Test Results
- Index
- Color Plates