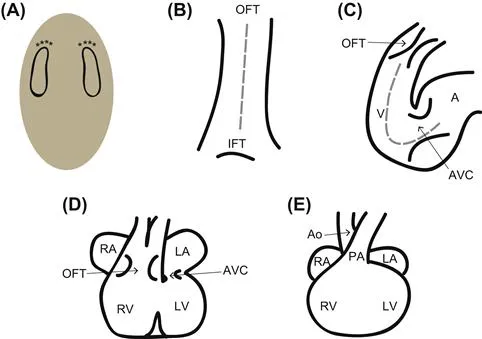
FIGURE 1.1 An overview of heart development. (A) The heart fields are specified as bilateral fields within the lateral plate mesoderm. The cranial-most aspect (asterisks) will migrate toward the midline first; this seam is depicted by the gray dashed line in B. (B) The heart tube closes ventrally, and cells continue to add from the heart fields. The dorsal aspect of the heart tube will pinch off last. After dorsal closure, additional cells can only be added via the venous (IFT, inflow tract) and arterial (OFT, outflow tract) poles. (C) As additional cells add to the heart tube, the heart tube begins to undergo looping, and the ventral midline becomes the outer curvature of the heart. During looping, the endocardial cushions begin forming in the atrioventricular canal and outflow tract. The atrioventricular canal separates the common atrium (A) from the common ventricle (V). (D) At the end of looping, the atrioventricular cushions are aligned dorsal to the outflow tract cushions, allowing connections between the left atrium and ventricle (LA and LV, respectively) and the right atrium and ventricle (RA and RV, respectively). As the outflow tract is septated, it also undergoes rotation to align the aorta with the left ventricle. Septa form between the ventricles and between the atria. (E) In the mature four-chambered heart, the aorta (Ao) serves as the outlet for the left side of the heart, and the pulmonary artery (PA) serves as the outlet for the right side of the heart.
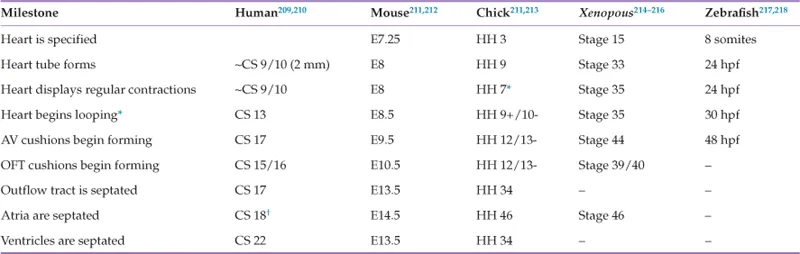
TABLE 1.1
Major Developmental Time Points in Humans and Common Experimental Models
The major stages in cardiovascular development are presented for humans and the most commonly used animal models.
∗Because the heart tube begins forming as a trough that then closes dorsally, contractions are observed prior to the pinching off of the fully formed heart tube.
†The foramen ovale is still open at this stage. This fenestration is closed at the stages listed for the other species. CS, Carnegie stage219; E, embryonic day; HH, Hamburger-Hamilton stage220; Stage, Nieuwkoop and Faber stage221; hpf, hours post-fertilization. See cited references for more details.
Signaling Pathways in Heart Field Specification
The Wnt Pathway
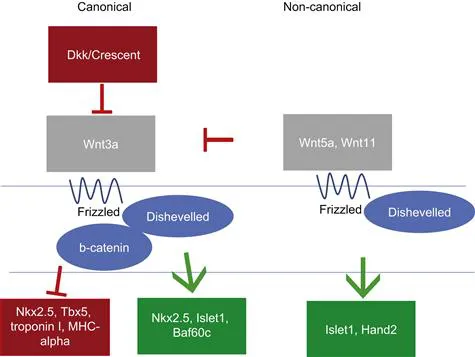
The Wnt family includes the canonical pathway, the non-canonical pathway (also known as the planar cell polarity pathway), and the Wnt/calcium pathway (Figure 1.2). Both the canonical and non-canonical pathways have well-established roles in heart field specification. Temporal waves of canonical and non-canonical Wnt signaling play distinct roles during cardiac specification and morphogenesis. As the heart field forms from the primitive streak, Wnt3a is expressed in the primitive streak and serves as a repulsive cue to the forming heart field.4 Experiments performed in Xenopus, due to its ease of manipulation and genetic tractability,5 have shown that the early repression of Wnt signaling in the Xenopus animal cap (i.e., in the ectodermal roof of the blastocele prior to heart field specification) via Dkk-1 and Crescent is necessary for initiation of transcription of cardiac transcription factors Nkx2.5 and Tbx5 and myocardial-specific proteins troponin I and myosin heavy chain α.6 However, later in cardiac development, canonical Wnt signaling in embryonic mice at E8.75 promotes Nkx2.5, Islet1, and Baf60c within the entire heart.7 Due to the genetic similarity between Xenopus and mouse, these differences likely reflect different temporal requirements for Wnt signaling as opposed to species-specific differences.5 In the second heart field, non-canonical Wnts 5a and 11, which act through the non-canonical planar cell polarity pathway, are expressed slightly later in development and co-operatively repress the canonical Wnt pathway while also promoting expression of Islet1 and Hand2, whose expression serves to ‘mark’ the heart field;8 as such, these genes are commonly referred to as cardiac markers. Both the repression of the canonical Wnts and the induction of the heart field markers require β-catenin in the second heart field.8 Wnt5a and Wnt11 also promote proliferation within this progenitor region.8 After the heart tube forms, Wnt-stabilized β-catenin is necessary in the Islet1-expressing second heart field cells to maintain their progenitor status.9 Loss of either β-catenin or Wnt signaling in the second heart field leads to second heart field defects, including right ventricular and outflow tract defects.9,10 Even if Wnt signaling is lost under cells expressing one of the first markers of differentiated cardiomyocytes, Mesp1, second heart field proliferation is decreased, and Islet-1 expression is down-regulated.11 Conversely, overexpressing β-catenin under the Mesp1 promoter expands the Islet-1-positive second heart field and promotes proliferation.11 Later, Wnt5a specifically acts upstream of the disheveled/planar cell polarity pathway to regulate the addition of the second heart field to the arterial pole.12 In addition, Wnt signaling also promotes bone morphogenetic protein (BMP) 4 and the non-canonical Wnt 11, which promote myocardial differentiation.9,11 Thus, the Wnt pathway is critical for inducing heart field formation, maintaining progenitor status and promoting myocardial differentiation.
FIGURE 1.2 Wnt signaling pathway. In both the canonical and non-canonical (planar cell polarity) pathways, extracellular Wnt ligands bind to the transmembrane receptor Frizzled. In the canonical pathway, Frizzled forms a complex with Dishevelled and additional components, leading to β-catenin stabilization and translocation to the nucleus; β-catenin then promotes Wnt-induced gene expression, such as Nkx2.5 and Islet1. In contrast, in the non-canonical pathway, Frizzled and Dishevelled act on a different set of intracellular signaling molecules (e.g., Rho, Rac) to promote a different set of genes and to inhibit the canonical pathway.
Retinoic Acid
One of the earliest required signaling pathways is the retinoic acid pathway. RALDH2, the enzyme that synthesizes retinoic acid, is restricted within the lateral plate mesoderm to a region nearest to the heart field.13,14 Expression of RALDH2 progresses in a cranial–caudal direction during heart field induction and heart tube formation and establishes the posterior boundary of the heart field.15 Within the lateral plate mesoderm, retinoic acid plays an inhibitory role, where it acts both directly and indirectly to restrict cardiac transcription factors Nkx2.5 and FoxF1 to the anterior lateral plate mesoderm, Hand1 to the anterior and middle of the lateral plate mesoderm, and Sal1 to the posterior lateral plate mesoderm.13,16,17 Retinoic acid further represses GATAs 4, 5, and 6.18 This inhibitory role is required to limit the size of the heart field, and fate-mapping studies in the zebrafish, another experimental model that is valued for its genetic similarity to the mouse and ease of studying embryonic development,19 have demonstrated that zebrafish embryos with decreased levels of retinoic acid exhibit an increased number of Nkx2.5-positive cells.17 Conversely, exposing either zebrafish or Xenopus embryos to increasing levels of retinoic acid specifically leads to a reduced number of cardiomyocytes.16,17 In chick embryos, which physically develop more similar to humans as compared with mice but lack the ability to manipulate the genome as in mice,20 antagonizing retinoic acid signaling promotes the ventricular myocardial fate at the expense of the atria.15 Together, these studies suggest that retinoic acid plays two major roles in the early heart field. First, retinoic acid generally restricts the expression of heart field markers to limit the size of the heart field. Then, it specifically promotes a ‘post...