
eBook - ePub
Handbook of Thermal Analysis and Calorimetry
Applications to inorganic and miscellaneous materials
- 942 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Handbook of Thermal Analysis and Calorimetry
Applications to inorganic and miscellaneous materials
About this book
This is the second volume of a four volume set intended to describe the techniques and applications of thermoanalytical and calorimetric methods. The general techniques and methodology are covered extensively in Volume 1, along with the fundamental physicochemical background needed. Consequently the subsequent volumes dwell on the applications of these powerful and versatile methods, while assuming a familiarity with the techniques.Volume 2 covers major areas of inorganic materials and some related general topics, e.g., catalysis, geochemistry, and the preservation of art. The chapters are written by established practitioners in the field with the intent of presenting a sampling of the how thermoanalytical and calorimetric methods have contributed to progress in their respective areas. The chapters are not intended as exhaustive reviews of the topics, but rather, to illustrate to the readers what has been achieved and to encourage them to consider extending these applications further into their domains of interest.- Provides an appreciation for how thermal methods can be applied to inorganic materials and processes.- Provides an insight into the versatility of thermal methods.- Shares the experiences of experts in a variety of different fields.- A valuable reference source covering a huge area of materials coverage.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Handbook of Thermal Analysis and Calorimetry by Michael E. Brown,Patrick K. Gallagher in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Analytic Chemistry. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
Applications of Thermal Analysis and Calorimetry in Adsorption and Surface Chemistry
Philip L. Llewellyn, MADIREL Laboratory, CNRS-Université de Provence, Marseille, France
1. Introduction
Several thermoanalytical and calorimetric methods can be used for the characterisation of adsorbent surfaces and of adsorption phenomena. The texture of solids, that is to say the extent of surface area and pore size distribution, can be characterised by controlled rate thermodesorption, thermoporometry and immersion calorimetry. An advantage of the calorimetric approach over more standard methods lies in the possibility to characterise a more realistic surface area in the case of microporous solids. The probing of the chemical nature of surface can also be attained by controlled rate thermodesorption and immersion calorimetry, as well as by adsorption calorimetry
The following paragraphs will briefly describe these methods and will highlight several results that have been obtained.
2. Immersion Calorimetry
2.1. Introduction
Immersion calorimetry is a simple method which can lead to information about the surface area as well as the surface chemistry of a solid. The surface area of a solid can be obtained by the immersion of the solid into a non-porous liquid or by using the modified Harkins and Jura method [1, 2]. In this method, the solid is pre-recovered with a film of liquid prior to immersion into the same liquid.
An estimation of a micropore size distribution can be obtained by the immersion into liquids of different molecular dimensions. Finally, the surface chemistry of a solid can be followed by the immersion into various polar liquids.
The experimental procedure is relatively straightforward. The sample cell is blown from glass with a brittle point added to the bottom of the bulb and the top open. After the sample is placed into the cell, it can be outgassed under vacuum in a standard manner or by using SCTA. The top of the cell is then closed and is attached to a glass rod which is found inside the immersion cane. The immersion fluid is added to a tube at the bottom of this cane. This tube can then be screwed on to complete the immersion cane. The whole system is placed into the calorimeter. After thermal equilibrium is attained, the glass rod is pressed down so that the brittle point on the sample cell is broken. This allows the intrusion of the immersion liquid to the sample and a heat effect is measured (Figure 1).
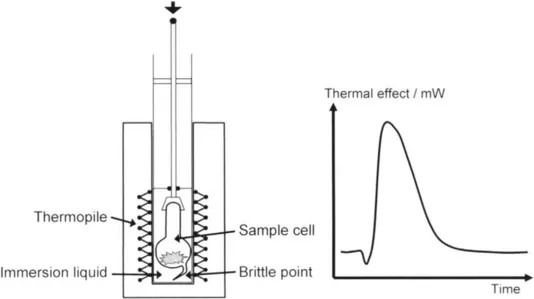
Figure 1 Schematic diagram of the set-up used for immersion calorimetry (left) and the thermal effect measured during an immersion experiment (right).
This heat effect, schematised in Figure 1, shows an initial dip before the peak. This endothermic dip corresponds to the initial vaporisation of the immersion liquid as it enters the cell. The exothermic peak then corresponds to the wetting of the sample, as well as to effects due to the breaking of the brittle point and the compression – depression of the immersion fluid inside and outside the cell. These different effects can be summarised by the following terms [2]:
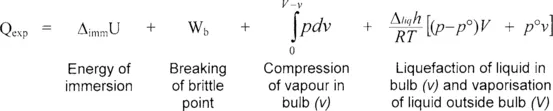
An estimation of the terms other than the energy of immersion can be obtained by a series of blank experiments with sample cells of various sizes. A plot of the heat effect measured as a function of the volume of the sample cell should then give a slope which is proportional to the heat of vaporisation of the immersion liquid.
The heat of immersion that is measured depends on several factors :
• The surface area of the solid. For solids of identical surface chemical nature, the immersion energy is proportional to the surface area. A means to overcome the problem of comparing solids with different surface chemistries is to use the modified Harkins and Jura method which is described below.
• The chemical nature of the surface. For a given liquid, the immersion energy depends of the chemical nature of the surface. For example, if the liquid is polar, the immersion energy increases with the polarity of the surface chemical functions. An application of such a study is to follow the influence of a treatment (thermal treatment, grafting …) on the nature and density of surface functions.
• The chemical nature of the liquid : for a given non-porous surface, the immersion enthalpy depends on the chemical nature of the immersion liquid. Here, an application can be the evaluation of the average dipolar moment of surface sites by immersion of the solid in liquids of increasing polarity. This leads to an analysis of the hydrophilic or hydrophobic character of a surface.
• The porosity. If the solid is microporous, the molecules of the liquid may be too large to penetrate into all the pores. In this case it is interesting to carry out a series of immersion experiments with molecules of different size but similar in chemical nature. In this case a micropore size distribution can be obtained and in some cases, it is possible to follow the kinetics of wetting or pore filling.
2.2. Immersion calorimetry for the estimation of the surface area of a solid: the modified Harkins and Jura method
As mentioned above, the surface area of a solid is proportional to the immersion energy that is released on wetting. In many cases it is possible immerge the solid into a non-polar liquid such as hexane. The heat effect measured can then be compared with that obtained with that of a reference solid.
In some cases though, the surface chemistry of the solid can still play a role on the heat effects measured. One procedure to overcome this problem is known as the modified Harkins and Jura method [2] which is schematised in Figure 2.
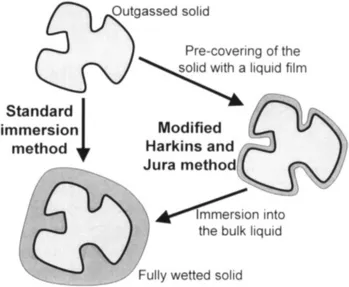
Figure 2 Schematic diagram comparing the standard immersion method with the modified Harkins and Jura method.
In this method, after outgassing, the sample is equilibrated at a given relative pressure of the immersion liquid. The surface is thus pre-covered with a liquid film prior to the immersion experimen...
Table of contents
- Cover image
- Title page
- Table of Contents
- Handbook of Thermal Analysis and Calorimetry
- Front Matter
- Copyright page
- Foreword
- Preface to Volume 2
- Contributors
- Acknowledgements
- Chapter 1: Applications of Thermal Analysis and Calorimetry in Adsorption and Surface Chemistry
- Chapter 2: The Applications of Thermoanalytical Techniques to The Preservation of Art and Archaeological Objects
- Chapter 3: The Application of Thermal Analysis to The Study of Carbons
- Chapter 4: Applications of Thermal Analysis in The Preparation of Catalysts and in Catalysis
- Chapter 5: Ceramics, Glass, and Electronic Materials
- Chapter 6: Thermal Analysis of Clays
- Chapter 7: Energy Storage
- Chapter 8: The Thermal Stability of Explosives
- Chapter 9: Fossil Fuels – Application of Thermal Analysis Techniques
- Chapter 10: General Inorganic Chemicals and Coordination Compounds
- Chapter 11: Applications of Thermal Methods in The Geosciences
- Chapter 12: Dehydration of Crystalline Hydrates
- Chapter 13: Thermal Analysis in Metallurgy
- Chapter 14: Pyrotechnics
- Chapter 15: Thermal Analysis in Studies Of High-Tc Superconductors
- Index