
eBook - ePub
Fish Physiology: Muscle Development and Growth
- 318 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Fish Physiology: Muscle Development and Growth
About this book
With the advent of zebrafish as a model system, the development and growth of muscle in fish has become an ever more important process. This volume, in the continuing Fish Physiology series, focuses attention on muscle from the genetics of muscle development to application of muscle growth patterns to aquacultural production.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Fish Physiology: Muscle Development and Growth by William S. Hoar,Anthony Farrell,Anthony P. Farrell in PDF and/or ePUB format, as well as other popular books in Sciences biologiques & Physiologie. We have over one million books available in our catalogue for you to explore.
Information
1
Induction and Patterning of Embryonic Skeletal Muscle Cells in the Zebrafish
Peter D. Currie and P.W. Ingham
I. Introduction
This chapter describes the embryonic origins and molecular events which control the formation of different populations of fish muscle cells. We focus primarily on myogenesis in the zebrafish and recent studies which have taken advantage of its simple embryology and genetic tractability to dissect the complex series of inductive cues and cell movements that underlie muscle cell specification. The optical clarity of the zebrafish embryo makes it especially well suited to the application of sophisticated cell labeling techniques, approaches that facilitate the direct visualization of muscle cell ontogeny. The identification of the genes that control this process is well underway following two large-scale mutant screens of the zebrafish genome; these have uncovered numerous mutants that disrupt muscle cell formation and differentiation in various ways (Driever et al., 1996; Haffter et al., 1996; van Eden et al., 1996; Granato et al., 1996). The combination of these two powerful approaches, together with strategies for analyzing the in vivo activities of cloned genes, is yielding an increasingly detailed understanding of the embryology of teleost muscle formation. Although the majority of the studies to date have focused on the mechanisms deployed in generating the axial musculature, we also briefly discuss the current state of understanding of the ways in which the other skeletal muscle populations, such as those present in the paired fins or attached to the embryonic cartilage of the segmented head skeleton, are generated. We chart the complex series of cell movements and behaviors that give rise to different populations of muscle cells and discuss what is known about the inductive cues that define these different populations.
II. The Process of Myogenesis in Zebrafish
A. Generating Somitic Muscle Precursors
In fish, as in other vertebrates, skeletal muscle of the trunk and tail derives from a specific embryological compartment, the myotome, which in amniotes has been shown to be induced within the segmented mesoderm of the somite. Somites condense from mesoderm immediately adjacent to the central body axis, the so-called paraxial mesoderm, and segment in a stereotypic rostral to caudal progression. Painstaking cell labeling and fate studies in zebrafish have shown that paraxial mesoderm derives from a specific region of the embryo identifiable just prior to gastrulation (Kimmel et al., 1990; Fig. 1). These cells undergo a complex series of cell movements before they initiate the myogenic program which is signaled by the onset of expression of the myogenic family of transcription factors.
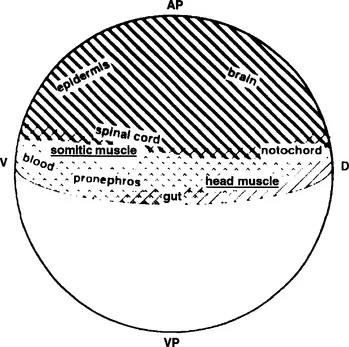
Fig. 1 Origin of zebrafish skeletal muscle cells in the early embryo. Cell labeling studies have revealed that the cells that are fated to give rise to zebrafish skeletal muscle (underlined) arise from a specific location within the zebrafish blastula. Reproduced from Kimmel et al. (1990), with the kind permission of the Company of Biologists.
The process in which cells, scattered around the hemisphere of the gastrulating zebrafish embryo, come to lie in axial and paraxial positions involves a set of cell movements termed convergence and extension. This process is central to the generation of the boundary between axial and paraxial populations and hence to the appropriate specification of muscle cells. Mutational analysis in the zebrafish has identified two genes that are critically required for the correct allocation of cells to the paraxial and axial mesoderm: the spadetail (spt) gene, which encodes a T-box transcription factor (Griffin et al., 1998), and the floating head (flh) gene, which encodes a homeodomain-containing transcription factor (Talbot et al., 1995). Activity of SPT is specifically required in the cells of the paraxial mesoderm, from which the axial musculature will derive. In the absence of SPT function, cells of the paraxial primordia migrate aberrantly during gastrulation and become associated with the tail, creating the characteristic “spadetail” after which the mutation was named (Ho and Kane, 1990; Warga et al., 1998). As a result, homozygous mutant spt embryos fail to form trunk somites and exhibit a severe lack of axial muscle (Kimmel et al., 1989). The myogenic transcription factor myoD (see below) also fails to be expressed during gastrulation and is very much reduced in expression during somitogenesis. Recent evidence has suggested that SPT may control the morphogenesis of paraxial mesoderm by regulating the differential expression of the cell adhesion molecule paraxial protocadherin (PAPC); (Yamamoto et al., 1998). The fact that PAPC function has been placed genetically downstream of SPT activity and the similarities in expression between the two genes suggest that SPT itself may directly regulate papc transcription. PAPC activity may in turn regulate the cell affinity differences between paraxial mesoderm precursors required for their correct migration.
The role of flh in mesoderm development is complementary to that of spt, being required for correct formation of the notochord, the most axial mesodermal fate. Embryos mutant for flh entirely lack notochord and instead form blocks of somitic muscle which fuse underneath the neural tube (Talbot et al., 1995). Lineage analysis has demonstrated that cells normally fated to become notochord differentiate instead into muscle in the absence of FLH activity; these cells initially express markers of both notochord and muscle differentiation (Halpern et al., 1995), but subsequently lose expression of the former. Consistent with this loss of function phenotype, overexpression of the Xenopus homolog of flh, XNot-2, in frog embryos results in excessive notochord formation (Gont et al., 1996). Taken together these results suggest that FLH acts to promote notochord development and repress muscle formation. Thus, the spatially regulated expression of spt and flh together creates the boundary between axial and paraxial mesoderm that is required for somite morphogenesis (Amacher et al., 1998).
B. Myotome Formation and Muscle Differentiation
Once cells have been allocated to either axial or paraxial mesoderm, the next phase of axial muscle specification begins. Cells become committed to the myogenic fate remarkably early in fish embryos, with the onset of expression of the myogenic transcription factor MyoD heralding restriction to the muscle lineages at the end of gastrulation. This contrasts greatly with the timing of initiation of myogenesis in amniote embryos, which does not occur until somitogenesis is well established. There is an obvious advantage for fish embryos, which develop from external fertilization, to generate the ability to move as quickly as possible after hatching in order to evade predation. By comparison amniotes have little need for the skeletal musculature until after birth.
In zebrafish, MyoD expression initiates in two triangular-shaped fields on either side of the forming axial midline (Weinberg et al., 1996). It is believed, but not proven, that the cells within this field converge to the midline during axis extension to generate a row of cells, termed the “adaxial cells,” which flank the forming notochord. This location adjacent to the notochord, as well their large cuboidal morphology, singles out these cells from the rest of the paraxial mesoderm (Figs. 2A and 2B, see color plate). The first elongating and striating cells of the teleost myotome, termed the muscle pioneers, arise from the adaxial cells (Waterman, 1969; Felsenfeld et al., 1991); these are clearly distinguishable from other muscle cells by their expression of the zebrafish engrailed (eng) 1 and 2 genes (Ekker et al., 1992; Fig. 2C). These cells differentiate in an anteroposterior wave mirroring somite differentiation, forming adjacent to the notochord, at the dorsoventral midline of the zebrafish myotome. At this level, a specialized structure of the myotome—the horizontal myoseptum—forms (Fig. 2D). Horizontal myosepta are present in all gnathostome fishes and divide the differentiating myotome into dorsal, nominally epaxial and ventral, nominally hypaxial muscle masses, but they are not found in cyclostomes (jawless fish; Bone, 1989). Structurally similar to vertical myosepta that separate adjacent mature myomeres in all vertebrates, horizontal myosepta are composed of connective tissue sheets...
Table of contents
- Cover image
- Title page
- Table of Contents
- Inside Front Cover
- Copyright page
- Contributors
- Preface
- Chapter 1: Induction and Patterning of Embryonic Skeletal Muscle Cells in the Zebrafish
- Chapter 2: Myogenic Regulatory Factors
- Chapter 3: Myosin Expression During Ontogeny, Post-Hatching Growth, and Adaptation
- Chapter 4: Muscle Satellite Cells in Fish
- Chapter 5: Cellular Mechanisms of Post-Embryonic Muscle Growth in Aquaculture Species
- Chapter 6: Genetic and Environmental Determinants of Muscle Growth Patterns
- Chapter 7: Muscle Fiber Diversity and Plasticity
- Chapter 8: Hormonal Regulation of Muscle Growth
- Index
- Other Volumes ln the Fish Physiology Series