
Global Clinical Trials
Effective Implementation and Management
- 522 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About this book
This book will explore the great opportunities and challenges which exist in conducting clinical trials in developing countries. By exploring the various regulations specific to the major players and providing insight into the logistical challenges including language barriers, this book provides a working tool for clinical researchers and administrators to navigate the intricacies of clinical trials in developing countries. Important topics such as ethical issues will be handled very carefully to highlight the significant differences of conducting this work in various jurisdictions. Overall, it will present a clear and comprehensive guide to the ins-and-outs of clinical trials in various countries to assist in design, development, and effectiveness of these trials.- Contributors include high-profile, respected figures who have paved the way for clinical trials in developing countries- Provides hands-on tools for regulatory and legal requirements and qualification, design, management, and reporting- Case studies outline successes, failures, lessons learned and prospects for future collaboration- Includes country-specific guidelines for the most utilized countries- Foreword by David Feigel, former Head of CDRH at FDA
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Richard Chin
Contents
1.1. Introduction
1.2. Growth of Global Clinical Trials
| These numbers are based on 121 applications with sufficient information to determine whether the data were non-US or US. | |||
| Marketing applications | Drugs | Biologicals | Drugs and biologicals |
|---|---|---|---|
| Applications with only US data | 15 | 9 | 24 |
| Applications with non-US and US data | 82 | 5 | 87 |
| Applications with only non-US data | 9 | 1 | 10 |
| Totals | 106 | 15 | 121 |
| These numbers are based on data from 193 clinical trials with complete subject and site information. | |||
| Drugs | Biologicals | Drugs and biologicals | |
|---|---|---|---|
| No. of non-US and US subjects | 92.859 | 206,842 | 299,701 |
| No. of non-US subjects | 52.820 | 179,712 | 232,532 |
| Percentage of non-US subjects | 56.9% | 86.9% | 77.6% |
| No. of non-US and US trial sites | 11,227 | 717 | 11,944 |
| No. of non-US trial sites | 6,129 | 356 | 6,485 |
| Percentage of non-US trial sites | 54.6% | 49.7% | 54.3% |
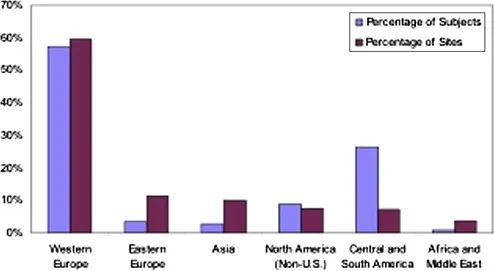 |
| Figure 1.1 Percentage of non-US clinical trial subjects and sites by region for FDA marketing applications approved in fiscal year 2008. These numbers are based on data from 193 clinical trials with complete subject and site information. Source: OIG analysis of FDA marketing applications approved in fiscal year 2008. |
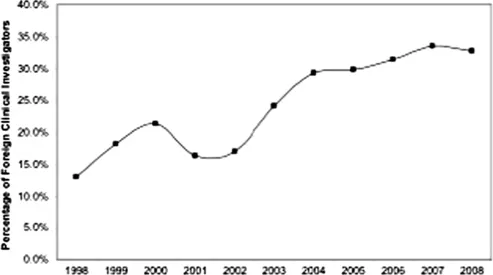 |
| Figure 1.2 Trend in non-US clinical investigators as a percentage of all clinical investigators identified in investigational new drug (IND) applications from 1998 to 2008. |
1.3. Drivers of Globalization
Table of contents
- Cover image
- Table of Contents
- Dedication
- Front Matter
- Copyright
- About the Editors
- Foreword
- Contributors
- Chapter 1. Background
- Chapter 2. Bioethical Considerations in Global Clinical Trials
- Chapter 3. United States Regulations
- Chapter 4. European Union Regulations
- Chapter 5. Japanese Regulations
- Chapter 6. Indian Regulatory Framework
- Chapter 7. Clinical Trials in India
- Chapter 8. Chinese Regulatory Framework
- Chapter 9. Clinical Trials in China
- Chapter 10. Clinical Trials in Taiwan
- Chapter 11. Clinical Trials in the Philippines
- Chapter 12. Clinical Trials in the Middle East and North Africa
- Chapter 13. Clinical Trials in South Africa
- Chapter 14. Clinical Trials in Latin America
- Chapter 15. Clinical Trials in Central and Eastern Europe
- Chapter 16. Design of Clinical Trials for Emerging Countries
- Chapter 17. Study Management
- Chapter 18. Study Documents and Logistics
- Chapter 19. Clinical Study Conduct and Monitoring
- Chapter 20. Data Collection, Data Management, and Electronic Data Capture
- Colorplates
- Index