
- 292 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Advances in Physical Organic Chemistry
About this book
Advances in Physical Organic Chemistry series is the definitive resource for authoritative reviews of work in physical organic chemistry. It aims to provide a valuable source of information not only for physical organic chemists applying their expertise to both novel and traditional problems, but also for non-specialists across diverse areas who identify a physical organic component in their approach to research. Its hallmark is a quantitative, molecular level understanding of phenomena across a diverse range of disciplines.- Reviews the application of quantitative and mathematical methods to help readers understand chemical problems- Provides the chemical community with authoritative and critical assessments of the many aspects of physical organic chemistry- Covers organic, organometallic, bioorganic, enzymes, and materials topics- Presents the only regularly published resource for reviews in physical organic chemistry- Written by authoritative experts who cover a wide range of topics that require a quantitative, molecular-level understanding of phenomena across a diverse range of disciplines
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Equilibrium Effective Molarity As a Key Concept in Ring-Chain Equilibria, Dynamic Combinatorial Chemistry, Cooperativity and Self-assembly
1 Corresponding authors: E-mail: [email protected]; [email protected]
Abstract
Keywords
1. Introduction
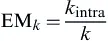
Table of contents
- Cover image
- Title page
- Table of Contents
- Advisory Board
- Copyright
- Contributors
- Preface
- Chapter One. Equilibrium Effective Molarity As a Key Concept in Ring-Chain Equilibria, Dynamic Combinatorial Chemistry, Cooperativity and Self-assembly
- Chapter Two. Thermodynamic Effective Molarities for Supramolecular Complexes
- Chapter Three. Reactivity of Nucleic Acid Radicals
- Chapter Four. Computational Studies of Environmental Effects and Their Interplay With Experiment
- Subject Index
- Author Index
- Cumulative Index of Titles
- Cumulative Index of Authors