
- 444 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Demand for fuel cell technology is growing rapidly. Fuel cells are being commercialized to provide power to buildings like hospitals and schools, to replace batteries in portable electronic devices, and as replacements for internal combustion engines in vehicles. PEM (Proton Exchange Membrane) fuel cells are lighter, smaller, and more efficient than other types of fuel cell. As a result, over 80% of fuel cells being produced today are PEM cells.
This new edition of Dr. Barbir's groundbreaking book still lays the groundwork for engineers, technicians and students better than any other resource, covering fundamentals of design, electrochemistry, heat and mass transport, as well as providing the context of system design and applications. Yet it now also provides invaluable information on the latest advances in modeling, diagnostics, materials, and components, along with an updated chapter on the evolving applications areas wherein PEM cells are being deployed.
- Comprehensive guide covers all aspects of PEM fuel cells, from theory and fundamentals to practical applications
- Provides solutions to heat and water management problems engineers must face when designing and implementing PEM fuel cells in systems
- Hundreds of original illustrations, real-life engineering examples, and end-of-chapter problems help clarify, contextualize, and aid understanding
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access PEM Fuel Cells by Frano Barbir in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Power Resources. We have over one million books available in our catalogue for you to explore.
Information
Chapter One
Introduction
1.1 What is a Fuel Cell?
A fuel cell is an electrochemical energy converter that converts chemical energy of fuel directly into direct current (DC) electricity. Typically, a process of electricity generation from fuels involves several energy conversion steps:
1. Combustion of fuel converts chemical energy of fuel into heat.
2. This heat is then used to boil water and generate steam.
3. Steam is used to run a turbine in a process that converts thermal energy into mechanical energy.
4. Finally, mechanical energy is used to run a generator that generates electricity.
A fuel cell circumvents all these processes and generates electricity in a single step without involving any moving parts (Figure 1-1). It is this simplicity that attracts attention. Such a device must be simpler, thus less expensive and far more efficient, than the four-step process previously depicted. Is it really? Today—not really! Or better, not yet. But fuel cells are still being developed. This book intends to provide a basis for engineering fuel cell devices. It includes state-of-the-art designs and materials (as they exist at the time of this writing), which are likely to change in the future as this technology continues to develop (perhaps even sooner than the students using this textbook get jobs in the fuel cell industry). However, the engineering basis will not change, at least not dramatically and not so quickly. The knowledge of engineering principles will allow future fuel cell engineers to adopt these new designs and new materials and, we hope, come up with even newer designs and materials. This is what the purpose of an engineering education should be. This book will not teach the principles of thermodynamics, catalysis, electrochemistry, heat transfer, fluid mechanics, or electricity conduction, but it will apply those engineering disciplines in the engineering of a fuel cell as an energy conversion device.
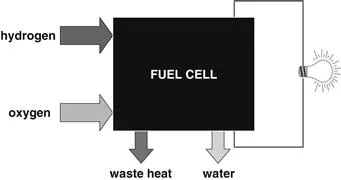
Figure 1-1 A fuel cell generates DC electricity from fuel in one step.
The efficiency of an energy conversion process is one of the most important aspects of that conversion. Some typical questions are usually tackled by engineering textbooks: How much energy of one kind is required to generate one unit of energy of another kind? What is the theoretical limit? How close can we come to that limit in practical applications? This last question is where most engineering textbooks fail in providing practical results and real—not theoretical—efficiencies.
As an example, let’s examine the Carnot process, also called the Carnot engine. Every engineering student should know that the Carnot process is the most efficient process to operate between given temperatures. The fact that such an engine cannot be made, and even if it could be made it would have to operate infinitesimally slowly to allow the heat transfer processes to happen with no losses, is perhaps mentioned in some textbooks. But the fact that such an engine would be very efficient at generating no power is never emphasized enough. Yes, the famous Carnot engine would have to operate at efficiencies lower than the famous Carnot efficiency in order to generate useful power. This book emphasizes the efficiency—not only the theoretical efficiency but the efficiency of practical, power-generating devices. That is why there is a subsection on efficiency in almost every chapter. The chapter on fuel cell thermodynamics deals with theoretical fuel cell efficiencies. As important as it is to learn about the Carnot efficiency, it is equally important to learn about theoretical limits in fuel cells. The chapter on fuel cell electrochemistry introduces various losses that are unavoidable because of the physical properties of the materials involved. These losses obviously have an effect on the efficiency of energy conversion. The chapter on fuel cell systems discusses various supporting devices that are needed to get the fuel cell going. Most of those devices need power, which means that some of the power produced by a fuel cell would be used to run those supporting devices, and therefore less net power would actually be delivered by the fuel cell system. This means that the practical efficiency will be somewhat lower than the theoretical one. How much lower? That would depend on the system configuration, design, and selection of auxiliary components. Finally, the efficiency of an energy conversion device in a practical application will probably depend on how that device is used. Does it run all the time at constant power output or does the power output vary? If it varies, how much and how often? These are the reasons that the efficiency is discussed in almost every chapter of this book.
Another important aspect of an energy conversion process is the cost. It is the cost of produced energy that matters in practical applications. Obviously, this cost depends greatly on the efficiency of the energy conversion process and the cost of the consumed (or, thermodynamically, the more correct term is converted) energy. The cost of the energy conversion device itself must also be taken into account. The cost of any device depends on the cost of the materials and the efforts (labor) involved to process those materials, make the components, and finally to assemble those components into a working device. Unfortunately, there is not enough information available on fuel cell costs, either materials or labor. One of the reasons the fuel cells are expensive is that they are not being mass produced; one of the reasons they are not being mass produced is that their markets are limited because they are expensive. This “chicken-and-egg” problem is typical for any new technology.
This book interweaves the theory and practice of the fuel cell and fuel cell system design, engineering, and applications. It does not provide a recipe on how to build the best possible fuel cell, but it gives an engineering student an understanding of the basic processes and materials inside a fuel cell. It also supplies enough tools and instructions on how to use them to design a fuel cell or a fuel cell system, or how to select a fuel cell for a particular application. This book does not provide a direct answer to all fuel cell-related questions, but it provides the engineering tools needed to find those answers.
A fuel cell is in some aspects similar to a battery. It has an electrolyte and negative and positive electrodes (Figure 1-2), and it generates DC electricity through electrochemical reactions. However, unlike a battery, a fuel cell requires a constant supply of fuel and oxidant. Also, unlike a battery, the electrodes in a fuel cell do not undergo chemical changes. Batteries generate electricity by the electrochemical reactions that involve the materials that are already in batteries. Because of this, a battery may be discharged, which happens when the materials that participate in the electrochemical reactions are depleted. Some batteries are rechargeable, which means that the electrochemical reactions may proceed in reverse when external electricity is applied—a process of recharging the battery. A fuel cell cannot be discharged as long as the reactants—fuel and oxidant—are supplied. Typical reactants for fuel cells are hydrogen and oxygen; however, neither has to be in its pure form. Hydrogen may be present either in a mixture with other gases (such as CO2, N2, and CO) or in hydrocarbons such as natural gas, CH4, or even in liquid hydrocarbons such as methanol, CH3OH. Ambient air contains enough oxygen to be used in fuel cells. Yet another difference between a fuel cell and a battery is that a fuel cell generates by-products—waste heat and water—and the system is required to manage those. (A battery also generates some heat but at a much lower rate that usually does not require any special or additional equipment.)
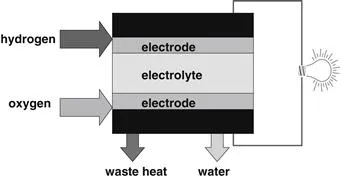
Figure 1-2 A fuel cell is similar to a battery in that it has electrodes and an electrolyte, but it needs a fuel and oxidant supply and it generates waste heat and water.
1.2 A Very Brief History of Fuel Cells
The timeline of fuel cell development history is shown in Figure 1-3. The first observation of a fuel cell effect was made by a German-Swiss scientist, Christian F. Shoenbein, in 1938 [1]. Apparently, based on this work, the first fuel cell was demonstrated by Welsh scientist and barrister Sir William Grove in 1839 [2]. In 1842, Grove developed the first fuel cell, or a gaseous voltaic battery, as he called it, which produced electrical energy by combining hydrogen and oxygen [3].
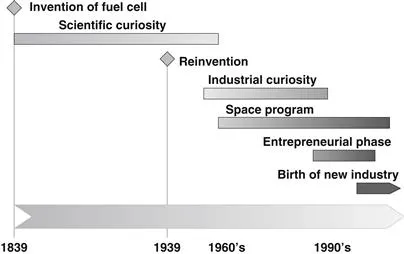
Figure 1-3 Fuel cell history timeline.
However, in spite of sporadic attempts to make a practical device, the fuel cell remained nothing more than a scientific curiosity for almost a century. In this period, W. F. Ostwald, a Nobel Prize winner in 1909 and founder of the field of physical chemistry, provided much of the theoretical understanding of how fuel cells operate. He realized that energy conversion in combustion engines is limited by the Carnot efficiency and results in unacceptable levels of atmospheric pollution, whereas the fuel cells, which directly generate electricity, are highly efficient, silent, and generate no pollution. He predicted a technical revolution, although realizing that practical realization of ...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Foreword
- Preface and Acknowledgments
- Preface to the Second Edition
- Chapter One. Introduction
- Chapter Two. Fuel Cell Basic Chemistry and Thermodynamics
- Chapter Three. Fuel Cell Electrochemistry
- Chapter Four. Main Cell Components, Material Properties, and Processes
- Chapter Five. Fuel Cell Operating Conditions
- Chapter Six. Stack Design
- Chapter Seven. Fuel Cell Modeling
- Chapter Eight. Fuel Cell Diagnostics
- Chapter Nine. Fuel Cell System Design
- Chapter Ten. Fuel Cell Applications
- Chapter Eleven. Durability of Polymer Electrolyte Fuel Cells
- Chapter Twelve. Future of Fuel Cells and Hydrogen
- Index