
- 1,028 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Electrochemical Sensor Analysis
About this book
Electrochemical Sensor Analysis (ECSA) presents the recent advances in electrochemical (bio)sensors and their practical applications in real clinical, environment, food and industry related samples, as well as in the safety and security arena. In a single source, it covers the entire field of electrochemical (bio)sensor designs and characterizations. The 38 chapters are grouped in seven sections: 1) Potentiometric sensors, 2) Voltammetric sensors, 3) Electrochemical gas sensors 4) Enzyme-based sensors 5) Affinity biosensors 6) Thick and thin film biosensors and 7) Novel trends. Written by experts working in the diverse technological and scientific fields related to electrochemical sensors, each section provides an overview of a specific class of electrochemical sensors and their applications.
This interdisciplinary text will be useful for researchers and professionals alike.
- Covers applications and problem solving (sensitivity, interferences) in real sample analysis
- Details procedures to construct and characterize electrochemical (bio)sensors
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Chapter 1 Clinical analysis of blood gases and electrolytes by ion-selective sensors
Publisher Summary
Electrochemical ion-selective sensors (ISSs) and amperometric gas-selective sensors (GSSs) have attracted the interest of clinical chemistry because they offer fast, reliable, inexpensive analytical results in service free automated analyzers. Now a days, the determination of blood gases (pO2, pCO2) is performed using electrochemical GSSs, while major blood electrolytes (Na+, K+as well as Ca2+, Mg2+, Li+ as a therapeutic ion and pH) and main urine electrolytes (Na+, K+) are predominantly determined by electrochemical ISSs or ISEs. Some important metabolites (glucose, lactate, urea, creatinine) are often determined with electrochemical biosensors. By taking into account the general properties of ISEs (concentration ranges, selectivity, expected life time, etc.) and by listing the ions of physiological importance, it is possible to deduce that some improvements or applications of new ISSs are still feasible. Further amelioration of magnesium electrodes is still likely because existing ones suffer from relatively poor selectivity.
1.1 Introduction
Electrochemical ion-selective sensors (ISSs), including potentiometric ion-selective electrodes (ISEs) and potentiometric or amperometric gas-selective sensors (GSSs), attracted the interest of clinical chemistry because they offer fast, reliable, inexpensive analytical results in service-free automated analyzers. In this way, the electrochemical sensors satisfy the present demands of central hospital laboratories and peripheral point-of-care medical service points, such as bedside, emergency or first-contact healthcare centers.
A breakthrough in the clinical application of electrochemical sensors came with blood gas analysis via pH (“glass electrode”[1]), pCO2 (“Severinghouse gas electrode”[2]) and pO2 (“Clark sensor”[3]) electrodes, introduced in the 1950s [4]. Another important milestone in this area was the invention of a valinomycin-based potassium ISE in 1970 [5]. The subsequent development of new generations of ISEs and ISSs, gas-selective electrodes and GSSs, supplemented recently with a rapidly growing family of electrochemical biosensors, provided analytical tools that permit the specific and precise determination of a number of species of medical importance.
Today the determination of blood gases (pO2, pCO2) is performed using electrochemical GSSs, while major blood electrolytes (Na+, K+, Cl− as well as Ca2+, Mg2+, Li+ as a therapeutic ion and pH) and main urine electrolytes (Na+, K+) are predominantly determined by electrochemical ISSs or ISEs. Some important metabolites (glucose, lactate, urea, creatinine) are often determined with electrochemical biosensors.
The application of ISSs/GSSs in clinical chemistry aroused notable interest in the chemical scientific community marked by dedicated reviews [6–11] and monographs [12–14], including a special series of reports devoted exclusively to the application of ISEs in clinical measurements [15]. For clinicians, normalized and traceable application of ISSs/GSSs in routine analysis is a professional obligation. For this reason their activity is supported by the dedicated activity of the International Federation of Clinical Chemistry (IFCC) and its Working Group on Selective Electrodes and Biosensors. Within this group a number of documents devoted to the application of electrochemical sensors in routine clinical chemistry were published [16–25].
1.2 General Characteristics of Clinical Analysis of Electrolytes and Gases
1.2.1 Clinical sample, analytical matrix
A clinical sample (whole blood, serum, plasma, urine, gastric juice, bile fluid, sweat, etc.) differs from any other analytical sample because of the presence of heterogeneous organic (e.g., proteins) and organic or inorganic components (e.g., urea or sodium ion), sample changes in time (owing to, e.g., denaturation of proteins, escape of CO2) and small sample size (even a few tens of microliters).
In the case of frequently measured whole blood samples, anaerobic blood samples (arterial or venous) are typically used for blood gas analysis, while blood plasma (i.e., the liquid component of blood, in which the blood cells are suspended, containing the clotting agent fibrinogen; the clotting process can be blocked by anticoagulants such as heparin) or blood serum (i.e., blood plasma not containing fibrinogen) are used for measurements of electrolytes and soluble molecules (e.g., glucose) dissolved in plasma water. Plasma water comprises approximately 80–96% of the total serum/plasma volume, with the remainder being composed of insoluble proteins, lipids and macromolecules. The normal water content in plasma is ∼93%. The major electrolytes contained in plasma water, i.e., sodium, potassium and chlorides, are nearly 100% dissociated. The terms “ionized”—sodium, potassium and chloride—are therefore used to describe the results obtained by ISE measurements in blood. Ionized calcium and magnesium refer to the free (unbound) fraction of total calcium (ca. 50%) and total magnesium (ca. 70%).
In the case of the frequently requested sample urine, the term “urine”—sodium and potassium—is applied. Urine has a different matrix than blood (chemical composition, ionic strength, interferences), which justifies the distinction in measuring terminology, although in both cases the same ISSs/ISEs are used.
The analytical ranges of major parameters in blood and urine are summarized in Table 1.1.
Table 1.1 Reference ranges for the adults’ clinical parameters measured by electrochemical sensors
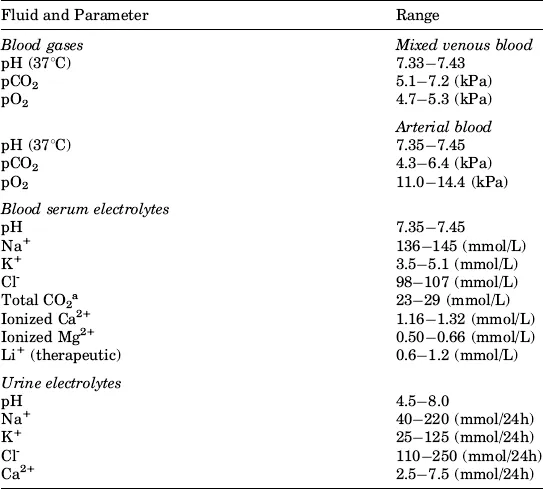
a HCO3− is ∼1.2 mmol/L lower.
The pathological values higher (“hyper”) or lower (“hypo”) are outside of the normal ranges given in Table 1.1, and consequently broader analytical ranges are set for individual sensors. Typical concentration ranges and within-run imprecision values are given in Table 1.2.
Table 1.2 Analytical ranges and imprecision of measurement in a typical blood gas and electrolyte analyzer
| Parameter | Analytical range | Within-run imprecision (SD) |
|---|---|---|
| pH | 6.50–8.00 | 0.005 |
| pCO2 (kPa) | 0.5–27 | 0.15 |
| pO2 (kPa) | 0–107 | 0.20 |
| Na+ (mmol/L) | 80–200 | 1.4 |
| K+ (mmol/L) | 2–10 | 0.05 |
| Cl− (mmol/L) | 50–150 | 1.0 |
| Total CO2 (mmol/L) | 5–40 | 0.5 |
| Ionized Ca2+ (mmol/L) | 0.2–3.0 | 0.02 |
| Ionized Mg2+ (mmol/L) | 0.2–2.0 | 0.02 |
| Na+ (urine) (mmol/L) | 20–300 | 5 |
| K+ (urine) (mmol/L) | 20–200 | 5 |
1.2.2 Types and design of ion-sensors and gas-sensors used in clinical analysis
The demand for reliability of the analyses, a long-life of the electrodes and their readiness to be used any time (for so-called Stat samples) directly dictated the design of ISSs/GSSs. Instead of macroelectrodes, a large variety of microelectrodes have been developed [26].
ISSs, as with any potentiometric sensor, can be treated as a galvanic half-cell and represented schematically:
Correspondingly the galvanic cell (with ISS) can be represented by the following scheme:

At present, most clinical measurements are realized in conti...
Table of contents
- Cover image
- Title page
- Table of Contents
- Contributors to Volume 49
- Volumes in the Series
- Editor's Preface
- Series Editor's Preface
- Chapter 1 Clinical analysis of blood gases and electrolytes by ion-selective sensors
- Chapter 2 Ion-selective electrodes in trace level analysis of heavy metals: Potentiometry for the XXI century
- Chapter 3 Enantioselective, potentiometric membrane electrodes: design, mechanism of potential development and applications for pharmaceutical and biomedical analysis.
- Chapter 4 Ion sensors with conducting polymers as ion-to-electron transducers
- Chapter 5 Light-addressable potentiometric sensors (LAPS): recent trends and applications
- Chapter 6 Stripping-based electrochemical metal sensors for environmental monitoring
- Chapter 7 Graphite-epoxy electrodes for stripping analysis
- Chapter 8 Voltammetric sensors for the determination of antioxidant properties in dermatology and cosmetics
- Chapter 9 Sensoristic approach to the evaluation of integral environmental toxicity
- Chapter 10 Peptide-modified electrodes for detecting metal ions
- Chapter 11 Reproducible electrochemical analysis of phenolic compounds by high-pressure liquid chromatography with oxygen-terminated diamond sensor
- Chapter 12 Chemical sensors for mercury vapour
- Chapter 13 Application of electrochemical enzyme biosensors for food quality control
- Chapter 14 Electrochemical biosensors for heavy metals based on enzyme inhibition
- Chapter 15 Ultra-sensitive determination of pesticides via cholinesterase-based sensors for environmental analysis
- Chapter 16 Amperometric enzyme sensors for the detection of cyanobacterial toxins in environmental samples
- Chapter 17 Electrochemical biosensors based on vegetable tissues and crude extracts for environmental, food and pharmaceutical analysis
- Chapter 18 Immunosensors for clinical and environmental applications based on electropolymerized films: analysis of cholera toxin and hepatitis C virus antibodies in water and serum
- Chapter 19 Genosensor technology for electrochemical sensing of nucleic acids by using different transducers
- Chapter 20 DNA-electrochemical biosensors for investigating DNA damage
- Chapter 21 Electrochemical genosensing of food pathogens based on graphite–epoxy composite
- Chapter 22 Electrochemical immunosensing of food residues by affinity biosensors and magneto sensors
- Chapter 23 Screen-printed electrochemical (bio)sensors in biomedical, environmental and industrial applications
- Chapter 24 Mediated enzyme screen-printed electrode probes for clinical, environmental and food analysis
- Chapter 25 Coupling of screen-printed electrodes and magnetic beads for rapid and sensitive immunodetection: polychlorinated biphenyls analysis in environmental samples
- Chapter 26 Thick- and thin-film DNA sensors
- Chapter 27 Screen-printed enzyme-free electrochemical sensors for clinical and food analysis
- Chapter 28 Analysis of meat, wool and milk for glucose, lactate and organo-phosphates at industrial point-of-need using electrochemical biosensors
- Chapter 29 Rapid detection of organophosphates, Ochratoxin A, and Fusarium sp. in durum wheat via screen printed based electrochemical sensors
- Chapter 30 Potentiometric electronic tongues applied in ion multidetermination
- Chapter 31 Electrochemical sensors for food authentication
- Chapter 32 From microelectrodes to nanoelectrodes
- Chapter 33 DNA/RNA aptamers: novel recognition structures in biosensing
- Chapter 34 Miniaturised devices: electrochemical capillary electrophoresis microchips for clinical application
- Chapter 35 Microchip electrophoresis/electrochemistry systems for analysis of nitroaromatic explosives
- Chapter 36 Microfluidic-based electrochemical platform for rapid immunological analysis in small volumes
- Chapter 37 Scanning electrochemical microscopy in biosensor research
- Chapter 38 Gold nanoparticles in DNA and protein analysis
- Subject Index
- Procedure 1 Measurement of ionized Mg2+ in human blood by ion-selective electrode in automatic blood electrolyte analyzer
- Procedure 2 Determination of cesium in natural waters using polymer-based ion-selective electrodes
- Procedure 3 Enantioanalysis of S-captopril using an enantioselective, potentiometric membrane electrode
- Procedure 4 Determination of Ca(II) in wood pulp using a calcium-selective electrode with poly(3,4-ethylenedioxythiophene) as ion-to-electron transducer
- Procedure 5 Titration of trimeprazine base with tartaric acid in isopropanol solution using polyaniline as indicator electrode
- Procedure 6 Determination of cadmium concentration and pH value in aqueous solutions by means of a handheld light-addressable potentiometric sensor (LAPS) device
- Procedure 7 Determination of lead and cadmium in tap water and soils by stripping analysis using mercury-free graphite–epoxy composite electrodes
- Procedure 8 Direct electrochemical measurement on skin surface using microelectrodes
- Procedure 9 Direct electrochemical measurements in dermo-cosmetic creams
- Procedure 10 Biosensor for integral toxicity
- Procedure 11 Photosensor of environmental permanence
- Procedure 12 Biosensors for the determination of radicals
- Procedure 13 The determination of metal ions using peptide-modified electrodes
- Procedure 14 Deposition of boron-doped diamond films and their anodic treatment for the oxygen-terminated diamond sensor
- Procedure 15 Chemoresistor for determination of mercury vapor
- Procedure 16 Determination of gluconic acid in honey samples using an integrated electrochemical biosensor based on self-assembled monolayer modified gold electrodes
- Procedure 17 Preparation of Prussian blue-modified screen-printed electrodes via a chemical deposition for mass production of stable hydrogen peroxide sensors
- Procedure 18 Electrochemical sensor array for the evaluation of astringency in different tea samples
- Procedure 19 Characterization of the PDO Asiago cheese by an electronic nose
- Procedure 20 Determination of methyl mercury in fish tissue using electrochemical glucose oxidase biosensors based on invertase inhibition
- Procedure 21 Protein phosphatase inhibition-based biosensor for amperometric microcystin detection in cyanobacterial cells
- Procedure 22 Voltammetric determination of paracetamol in pharmaceuticals using a zucchini (Cucurbita pepo) tissue biosensor
- Procedure 23 Determination of total phenols in wastewaters using a biosensor based on carbon paste modified with crude extract of jack fruit (Artocarpus integrifolia L.)
- Procedure 24 Construction of an enzyme-containing microelectrode array and use for detection of low levels of pesticides
- Procedure 25 PCB analysis using immunosensors based on magnetic beads and carbon screen-printed electrodes in marine sediment and soil samples
- Procedure 26 Construction of amperometric immunosensors for the analysis of cholera antitoxin and comparison of the performances between three different enzyme markers
- Procedure 27 Electrochemical detection of calf thymus double-stranded DNA and single-stranded DNA by using a disposable graphite sensor
- Procedure 28 Atomic force microscopy characterization of a DNA electrochemical biosensor
- Procedure 29 Electrochemical sensing of DNA damage by ROS and RNS produced by redox activation of quercetin, adriamycin and nitric oxide
- Procedure 30 Electrochemical determination of Salmonella spp. based on GEC electrodes
- Procedure 31 Rapid electrochemical verification of PCR amplification of Salmonella spp. based on m-GEC electrodes
- Procedure 32 In situ DNA amplification of Salmonella spp. with magnetic primers for the real-time electrochemical detection based on m-GEC electrodes
- Procedure 33 Electrochemical determination of atrazine in orange juice and bottled water samples based on Protein A biocomposite electrodes
- Procedure 34 Electrochemical determination of sulfonamide antibiotics in milk samples using a class-selective antibody
- Procedure 35 Preparation of electrochemical screen-printed immunosensors for progesterone and their application in milk analysis
- Procedure 36 Genosensor on gold thin-films with enzymatic electrochemical detection of a SARS virus sequence
- Procedure 37 Genosensor on streptavidin-modified thick-film carbon electrodes for TNFRSF21 PCR products
- Procedure 38 Electrochemical immunosensor for diagnosis of the forest-spring encephalitis
- Procedure 39 Non-enzymatic urea sensor
- Procedure 40 Potentiometric determination of antioxidant activity of food and herbal extracts
- Procedure 41 Convenient and rapid detection of cholinesterase inhibition by pesticides extracted from sheep wool
- Procedure 42 Detection of dichlorvos in durum wheat
- Procedure 43 Detection of pirimiphos-methyl in durum wheat
- Procedure 44 Detection of Fusarium sp. via electrochemical sensing
- Procedure 45 An electronic tongue made of coated wire potentiometric sensors for the determination of alkaline ions: Use of artificial neural networks for its response model
- Procedure 46 Determination of gold by anodic stripping voltammetry in tap water
- Procedure 47 Detection of the aptamer–protein interaction using electrochemical indicators
- Procedure 48 Separation and amperometric detection of hydrogen peroxide and L-ascorbic acid using capillary electrophoresis microchips
- Procedure 49 Analysis of nitroaromatic explosives with microchip electrophoresis using a graphite–epoxy composite detector
- Procedure 50 Determination of sub-pM concentration of human interleukin-1B by microchip ELISA with electrochemical detection
- Procedure 51 Kinetic analysis of titanium nitride thin films by scanning electrochemical microscopy
- Procedure 52 Analysis of the activity of β-galactosidase from E. Coli by scanning electrochemical microscopy (SECM)
- Procedure 53 DNA analysis by using gold nanoparticle as labels
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Electrochemical Sensor Analysis by Salvador Alegret,Arben Merkoci in PDF and/or ePUB format, as well as other popular books in Biological Sciences & Biotechnology. We have over one million books available in our catalogue for you to explore.