
- 387 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Alkaloids: Chemical and Biological Perspectives
About this book
Acronycine, a potent antitumor agent, was discovered in the bark of the small Australian Rutaceous tree, Acronychia baueri Schott. This new work presents a comprehensive survey of the isolation, structure determination, methods of synthesis, and the biological properties of acronycine, as well as an account of natural and synthetic analogues of acronycine, and their biological properties.Solanum alkaloids were reviewed in 1990 and this book surveys the new developments (isolation procedures, structural elucidation methods) and critically updates earlier reviews. In addition it presents the interesting chemistry and synthesis of cyclopeptide alkaloids. These cyclopeptide alkaloids have been isolated from ascidians, sea hares, and cyanobacteria. Also included are reviews of the use of the functionalized lactam, pyroglutamic acid, as a chiral template for the synthesis of alkaloids. The second review examines the on-line coupling of capillary electrophoresis (CE) and mass spectrometry (MS) for the analysis of alkaloid mixtures. Finally a review of oxygenated analogs of the alkaloid Marcfortine for their potent antiparasitic activity is included at the end of this work. Each chapter in this volume has been reviewed by at least one expert in the field. Indexes for both subjects and organisms are provided.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Alkaloids: Chemical and Biological Perspectives by S.W. Pelletier in PDF and/or ePUB format, as well as other popular books in Sciences physiques & Biochimie. We have over one million books available in our catalogue for you to explore.
Information
Chapter One
Acronycine-Type Alkaloids : Chemistry and Biology
François Tillequin; Sylvie Michel; Alexios-Léandros Skaltsounis Université René Descartes - Paris V, Faculté de Pharmacie, Laboratoire de Pharmacognosie, 75270 Paris Cédex 06, France
1 INTRODUCTION
Acronycine (3,12-dihydro-6-methoxy-3,3,12-trimethyl-7H-pyrano[2,3-c]acridin-7-one) (1) is a natural alkaloid which was first isolated in 1948 by the group of Hughes and Lahey [1] from the bark of the small Australian Rutaceous tree Acronychia baueri Schott.
The structure of acronycine has long been discussed, mainly to ascertain whether the pyran ring was fused linearly or angularly on the acridone skeleton. It was only in 1966 that the angular structure 1 could be unambiguously assigned to acronycine on the basis of oxidative degradation evidence [2] and of 1H nmr data [3]. Final proof of the structure was obtained in 1970 from X-ray crystallographic data of 5-bromo-1,2-dihydroacronycine (2) [4].
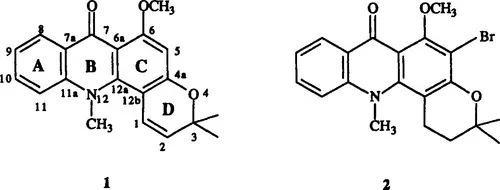
The biological interest of acronycine was revealed in 1966 by Svoboda and co-workers in the Eli Lilly Laboratories [5, 6]. Acronycine is a potent antitumor agent whose main interest lies in its broad spectrum of activity, including numerous solid tumors resistant to other chemotherapeutic agents [5–8]. In contrast, acronycine exhibits only marginal activity against leukemias [5–8].
Since the discovery of the antitumor properties of acronycine, numerous derivatives and structural analogues have been both isolated from various Rutaceae species and prepared by total synthesis. A survey of natural alkaloids and synthetic analogues derivating from the pyrano[2,3-c]acridin-7-one skeleton is presented below.
2 ACRONYCINE
2.1 Isolation, Chemical Properties and Structural Elucidation
Isolation. The first isolation of acronycine from a methanolic extract of Acronychia baueri bark relied only on solubility differences between the various alkaloids contained in the plant material [9]. Further isolations from the same source involved both crystallizations and chromatography on alumina and/or silica gel [5, 6, 10]. A chemical study of the leaves of the same plant resulted in the isolation of various acridone and furo[2,3-b]quinoline alkaloids but no acronycine could be detected [11].
Since the first isolation of acronycine, the status of Acronychia baueri Schott within the Rutaceae family has been revised several times by Hartley at the Herbarium Australiense, in the course of successive taxonomic studies of genera Acronychia [12], Bauerella [13] and Sarcomelicope [14, 15]. Hartley now considers this taxon belongs to the genus Sarcomelicope and should be named Sarcomelicope simplicifolia (Endl.) Hartley subsp. simplicifolia [14].
Apart from Sarcomelicope simplicifolia, all the other species belonging to that genus are endemic in New Caledonia [14, 15], and most of them have been studied for their alkaloid contents [16–27]. Acronycine was obtained from the bark of Sarcomelicope simplicifolia (Endl.) Hartley subsp. neo-scotica (P.S. Green) Hartley [16, 18], from the bark of Sarcomelicope argyrophylla Guill. [20], from the bark of Sarcomelicope glauca Hartley [21], and from the leaves and bark of Sarcomelicope dogniensis Hartiey [22, 25] and Sarcomelicope pembaiensis Hartley [23]. In addition, acronycine has also been isolated from the aerial parts of Melicope leptococca (Baill.) Guill. [28].
Chemical properties and structural elucidation. Acronycine crystallizes from alcohol as yellow needles, m.p. 175-176 °C [9]. It also readily crystallizes from methanol and acetone [5]. A dilute alcoholic solution of the base is yellow with a bright green fluorescence [9]. Acronycine forms an orange picrate, m.p. 150-154 °C, a red hydrochloride which easily separates from 10 per cent hydrochloric acid in red needles, m.p. 125-130 °C (dec.), and a sulfate which crystallizes as red needles from alcoholic sulfuric acid, m.p. 158-159 °C [9].
Treatment of acronycine (1) with hot alcoholic hydrochloric acid yields polymeric amorphous products. In contrast, heating acronycine hydrochloride in the dry state brings demethylation with the formation of noracronycine (3) [29] (Scheme 1). The hydroxy group of this latter compound is chelated by the neighbouring carbonyl group at C(7), as shown by uv spectroscopy [30] and therefore cannot be methylated upon treatment with diazomethane [29]. However, treatment with dimethylsulfate and potassium carbonate in acetone converts it b...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright page
- Dedication
- Contributors
- Preface
- Contents of Previous Volumes
- Chapter One: Acronycine-Type Alkaloids : Chemistry and Biology
- Chapter Two: Solanum Steroid Alkaloids - an Update
- Chapter Three: Synthesis and Structure-Activity Studies of Lissoclinum Peptide Alkaloids
- Chapter Four: Pyroglutamate as a Chiral Template for the Synthesis of Alkaloids
- Chapter Five: Analysis of Alkaloids by Capillary Electrophoresis and Capillary Electrophoresis - Electrospray Mass Spectrometry
- Chapter Six: Oxidation of Anthelmintic Marcfortine A, an Indole Alkaloid
- Subject index
- Organism index