
eBook - ePub
Chemometrics
A Textbook
- 500 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Most chemists, whether they are biochemists, organic, analytical, pharmaceutical or clinical chemists and many pharmacists and biologists need to perform chemical analysis. Consequently, they are not only confronted with carrying out the actual analysis, but also with problems such as method selection, experimental design, optimization, calibration, data acquisition and handling, and statistics in order to obtain maximum relevant chemical information. In other words: they are confronted with chemometrics. This book on chemometrics, written by some of the leaders in the field, aims to provide a thorough, up-to-date introduction to this subject. The reader is given the opportunity to acquaint himself with the tools used in this discipline and the way in which they are applied. Some practical examples are given and the reader is shown how to select the appropriate tools in a given situation.As such the book provides the means to approach and solve analytical problems strategically and systematically, without the need for the reader to become a fully-fledged chemometrician. The authors' aim was to write a tutorial book which would be useful to readers at every level in this field.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Chapter 1
Chemometrics and the Analytical Process
1 Definition of chemometrics
The subject matter of this book is chemometrics, a term coined in 1972, which can be defined as the chemical discipline that uses mathematical, statistical, and other methods employing formal logic (a) to design or select optimal measurement procedures and experiments, and (b) to provide maximum relevant chemical information by analyzing chemical data.
Chemometrics has found widespread application in analytical chemistry and therefore that, essentially, is what this book is about. At the same time, it is also a book about the essentials of analytical chemistry. If one leaves out the words mathematical, etc. from the definition, one observes that chemometrics is really about what all analytical chemists try to do, namely to design optimal analytical procedures and to try and obtain as much information as possible from the results. Since chemometrics does this with the help of mathematical methods, it has evolved to the theoretical cornerstone of what we will call the analytical process, i.e. the reasoning followed by the analyst to select and optimize procedures, to carry them out in an efficient way, and to interpret the results correctly with a maximum of relevant information as the end product.
2 The intelligent laboratory concept
Figure 1 gives a general picture of the analytical process. Further detail is given in Fig. 2.
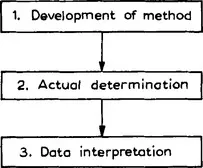
Fig. 1 The main steps in the analytical process. Chemometrics is concerned only with steps 1 and 3.

Fig. 2 A more detailed view of the analytical process. The numbers in the boxes relate to Fig. 1.
The analytical process starts with a problem that has to be solved and to solve it one needs chemical information. It is the task of the analytical chemist to provide this. His first step will be to select a method. Let us suppose he has to know the quality of a certain foodstuff and to do this he needs to determine the trace element content. He then has to decide whether he will use atomic absorption spectrometry (AAS), neutron activation analysis, inductively coupled plasma emission, or some other method. If he chooses AAS, his next decision will then be to decide on a flame or flameless method and if he selects the latter, what ashing temperature or gradient would be indicated. He will also have to decide on the pretreatment of the sample (wet ashing or low-temperature ashing, extraction or not, what kind of extraction, etc). All this will lead him to an initial procedure. Very probably, this procedure will not be the final one. The analytical chemist then tries to optimize the initial procedure by experimental optimization. He changes the pH of the buffer to obtain a more complete extraction and the drying temperature in the oven to obtain more reproducible results.
The procedure is now available and the analysis can begin. This is usually divided into two parts, the pretreatment and the actual determination. The pretreatment consists of operations such as weighing, extracting, drying, centrifugation, etc. This step is often the most difficult and time-consuming and determines the quality and efficiency of the method.
The result of the determination is a (usually electrical) signal and nowadays it is retrieved from the instrument by a computer. Very often, this signal is first treated to make it more useful by, for instance, reducing noise and it is then translated into chemical information. This means that a list of chemical identities and concentrations is now obtained. To achieve this translation, models describing the relationship between signal and concentration or identity, such as calibration models, are required. Some analysts finish here but one should remember, of course, that the analysis was carried out to solve a problem. This means that the chemical information should be translated into user or diagnostic information. Is the foodstuff acceptable for consumption?; does the analysis of an air sample indicate that a certain industry is responsible for air pollution at the collection point?; does the result of a patient’s blood tests indicate a certain disease?, etc. While the answer may be simple in some cases (for example, the foodstuff contains chemical X in excess of dose Y and therefore violates legal rules) it may be much more complex in certain cases and necessitate the application of certain mathematical techniques.
The analytical process described in this way can be considered as a system regulated by two feedback loops (Fig. 3). The first, internal to the laboratory, is the quality evaluation loop. Its purpose is to verify whether the performance of the method is good enough to achieve the analytical purpose for which it was developed and carried out. This loop requires the definition and evaluation of performance criteria (is the method “good” enough?) and the development of quality control schemes (does the method remain good enough when it is carried out repeatedly or continuously over a period of time?).
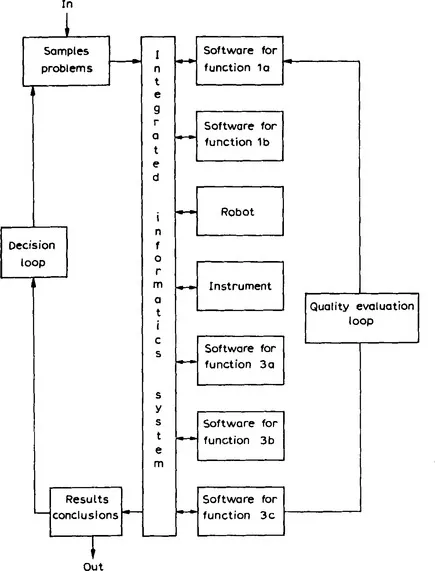
Fig. 3 The analytical process and its environment. The numbers and letters in the boxes relate to Fig. 2.
The second loop (the decision loop in Fig. 3) is the interaction with the outside world. The analytical results (hopefully) serve to solve a problem for, or to make a decision by, the person or organisation that asked for the results in the first place. This usually leads to new questions or, when the results did not bring the expected solution to the problem, to a better formulation of the question. In many instances, the analytical results also serve to control some process and the characteristics of the process determine the required characteristics of the analytical method.
Chemometrics is involved in steps 1a, 1b, 3a, 3b and 3c and in both control loops. Practical chemometrics is a matter of carrying out computations and this means that, in each of these steps, a computer is involved. This is certainly true also for steps 2a and 2b. More and more instruments are now attached to a computer (2a) and robots (2b), which are really computers with a hand, are often used to carry out the pretreatment step. In fact, we conclude that all the steps of Fig. 2 are computer-compatible. It is our belief that the separate functions of Figs. 2 and 3 will slowly be integrated and controlled by a central laboratory information system. When this integration has been achieved, an intelligent laboratory will have been developed. It will be able to select and optimize a procedure by itself, carry it out, extract the relevant information, check its own good functioning, and help in making decisions.
The integrated intelligent laboratory described in this way will rely heavily on software and the purpose of this book is to give the formal and mathematical background of the algorithms and techniques used. In fact, an alternative definition of chemometrics in analytical chemistry could be that it is the chemical discipline that studies mathematical, statistical and other methods employing formal logic to achieve the development of an integrated intelligent laboratory as described in Fig. 3. The technical software problems are not discussed. For instance, although we consider that robotics may become an important part of the intelligent laboratory, its development is mainly a question of hardware and software technology and therefore there will be no chapter on robotics in this book. There is also a lot of interest nowadays in expert systems. These will certainly be of use in those steps where the analytical chemist uses expertise, such as in the development of the analytical procedure or in the interpretation of spectroscopic data (structural analysis). However, again, this is mainly a problem of software and knowledge engineering, which we believe to be beyond the scope of a textbook on chemometrics today.
3 Organization of the book
The mathematical techniques and the formal concepts which must be introduced, unfortunately cannot be ordered in the same way as in Figs. 1-3. Because the first thing one really does when one needs to develop a new procedure is to define its desired characteristics, we started the book with a discussion on performance characteristics. Indeed, before it is possible to make a selection of a method or to carry out an optimization, one must have criteria according to which this may be done. Consequently, the performance of analytical procedures has to be evaluated by determining one or more performance characteristics of the procedure. The set of criteria has to be defined for each problem and will include quantities such as precision, accuracy, limits of detection and interferences. Up to now, most of these criteria have been used in quantitative analysis and it is probable that another set of characteristics will...
Table of contents
- Cover image
- Title page
- Table of Contents
- Inside Front Matter
- Front Matter
- Copyright page
- Introduction
- Chapter 1: Chemometrics and the Analytical Process
- Chapter 2: Precision and Accuracy
- Chapter 3: Evaluation of Precision and Accuracy. Comparison of Two Procedures
- Chapter 4: Evaluation of Sources of Variation in Data. Analysis of Variance
- Chapter 5: Calibration
- Chapter 6: Reliability and Drift
- Chapter 7: Sensitivity and Limit of Detection
- Chapter 8: Selectivity and Specificity
- Chapter 9: Information
- Chapter 10: Costs
- Chapter 11: The Time Constant
- Chapter 12: Signals and Data
- Chapter 13: Regression Methods
- Chapter 14: Correlation Methods
- Chapter 15: Signal Processing
- Chapter 16: Response Surfaces and Models
- Chapter 17: Exploration of Response Surfaces
- Chapter 18: Optimization of Analytical Chemical Methods
- Chapter 19: Optimization of Chromatographic Methods
- Chapter 20: The Multivariate Approach
- Chapter 21: Principal Components and Factor Analysis
- Chapter 22: Clustering Techniques
- Chapter 23: Supervised Pattern Recognition
- Chapter 24: Decisions in the Analytical Laboratory
- Chapter 25: Operations Research
- Chapter 26: Decision Making
- Chapter 27: Process Control
- Appendix
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Chemometrics by S.N. Deming,Y. Michotte,D.L. Massart,L. Kaufman,B.G.M. Vandeginste in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Analytic Chemistry. We have over one million books available in our catalogue for you to explore.