![]()
Chapter 1
Survey of the Problem and Certain Definitions
Nowadays, we are witnessing the development and advancement of a new interdisciplinary scientific field—nanoscience. Despite its name, it cannot be associated solely with miniaturization of the studied objects. In fact, nanoscience comprises closely interrelated concepts of chemistry, physics, and biology, which are aimed at the development of a new fundamental knowledge. As was shown by numerous examples in physics, chemistry, and biology, a transition from macrosizes to those of 1–10 nm gives rise to qualitative changes in physicochemical properties of individual compounds and systems.
The historical aspect of the formation and development of independent fundamental directions of nanoscience and the prospects of their application in different branches of nanotechnology were discussed in detail in numerous reviews.1–4 Numerous books and articles by Russian scientists who had a great influence on the progress in studying small-scale particles and materials can be found in Ref. 3. Their contribution was acknowledged to a certain extent by the 2000 Nobel Prize, which was awarded to Zh. I. Alferov for his achievements in the field of semiconducting heterostructures.
In the past 10–15 years, the progress in nanoscience was largely associated with the elaboration of new methods for synthesizing, studying, and modifying nanoparticles and nanostructures. The extensive and fundamental development of these problems was determined by nanochemistry. Nanochemistry, in turn, has two important aspects. One of these is associated with gaining insight into peculiarities of chemical properties and the reactivity of particles comprising a small number of atoms, which lays new foundations of this science. Another aspect, connected to nanotechnology, consists of the application of nanochemistry to the synthesis, modification, and stabilization of individual nanoparticles and also for their directed self-assembling to give more complex nanostructures. Moreover, the possibility of changing the properties of synthesized structures by regulating the sizes and shapes of original nanoparticles deserves attention.
The advances in recent studies along the directions mentioned are reflected in several reviews and books.5–13 A special issue of the journal Vestnik Moskovskogo Universiteta was devoted to the problems of nanochemistry.14 The dependence of physicochemical properties on the particle size was discussed based on optical spectra,15 magnetic properties,16,17 thermodynamics,18 electrochemistry,19 conductivity, and electron transport.20,21 Different equations describing physical properties as a function of the particle size were derived within the framework of the droplet model.22 A special issue of Journal of Nanoparticle Research is devoted to the works of Russian investigators in the field of nanoscience.23 Many aspects of synthesis, physicochemical properties, and self-assembly have been reviewed.24
In nanochemistry, which is in a stage of rapid development, questions associated with definitions and terms still arise. The exact difference between terms such as “cluster,” “nanoparticle,” and “quantum dot” has not yet been formulated in the literature. The term “cluster” is largely used for particles that include small numbers of atoms, while the term “nanoparticle” is applied for larger atomic aggregates, usually when describing the properties of metals and carbon. As a rule, the term “quantum dot” concerns semiconductor particles and islets, the properties of which depend on quantum limitations on charge carriers or excitons. In this book, no special significance will be attached to definitions, and the terms “cluster” and “nanoparticle” will be considered as interchangeable.
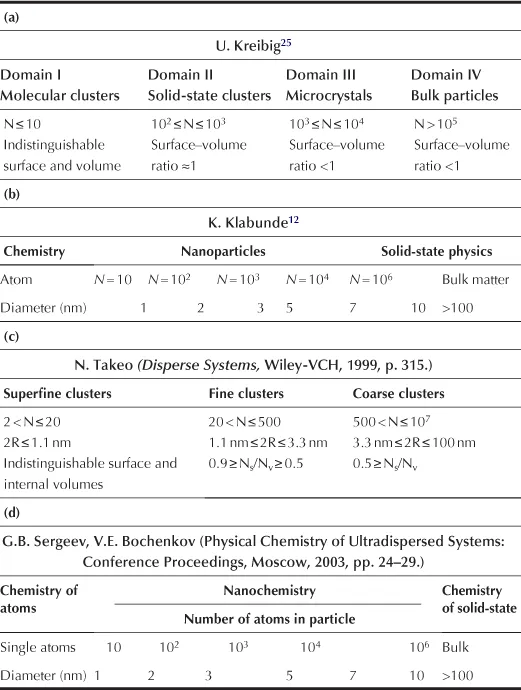
Table 1.1 shows some classifications of nanoparticles, which were proposed by different authors based on the diameter of a particle expressed in nanometers and the number of atoms in a particle. These classifications also take into account the ratio of surface atoms to those in the bulk. A definition given by Kreibig25 is similar to that proposed by Gubin.26 It should be mentioned that a field of chemistry distinguished by Klabunde12 pertains, in fact, to particles measuring less than 1 nm.
TABLE 1.1
Classification of Particles by their Sizes
Nanoparticles and metal clusters represent an important state of condensed matter. Such systems display many peculiarities and physical and chemical properties that were never observed earlier. Nanoparticles may be considered as intermediate formations, which are limited by individual atoms on the one hand and the solid phase on the other. Such particles exhibit the size dependence and a wide spectrum of properties. Thus, nanoparticles can be defined as entities measuring from 1 to 10 nm and built of atoms of one or several elements. Presumably, they represent closely packed particles of random shapes with a sort of structural organization. One of the directions of nanoscience deals with various properties of individual nanoparticles. Another direction is devoted to studying the arrangement of atoms within a structure formed by nanoparticles. Moreover, the relative stability of individual parts in this nanostructure can be determined by variations in kinetic and thermodynamic factors. Thus, nanosystems are characterized by the presence of various fluctuations.
Natural and technological nanoobjects represent, as a rule, multicomponent systems. Here again, one is up against a large number of different terms such as “nanocrystal,” “nanophase,” “nanosystem,” “nanostructure,” and “nanocomposites,” which designate formations built of individual, separate nanoparticles. For instance, nanostructure can be defined as an aggregate of nanoparticles of definite sizes, which is characterized by the presence of functional bonds. In the reactions with other chemical substances, such limited-volume systems can be considered as a sort of nanoreactors. Nanocomposites represent systems where nanoparticles are packed together to form a macroscopic sample in which interactions between particles become strong, masking the properties of individual particles. For every type of interaction, it is important to know how the properties of a sample change with its size. Moreover, it should be mentioned that with a decrease in the particle size, the concept of phase becomes less clear: it is difficult to find boundaries between homogeneous and heterogeneous phases, and between amorphous and crystalline states. At present, the common concepts of chemistry, which define the relationships such as composition–properties and structure–function, are supplemented by the concepts of size and self-organization, giving rise to new effects and mechanisms. Nonetheless, despite all achievements of nanochemistry, we still cannot give a general answer to the question how the size of particles of, e.g. a metal, is related to their properties.
Metallic nanoparticles measuring less than 10 nm represent systems with excessive energy and a high chemical activity. Particles of about 1 nm need virtually no activation energy to enter into either aggregation processes, which result in the formation of metal nanoparticles, or reactions with other chemical compounds to give substances with new properties. The stored energy of such particles is determined first of all by uncompensated bonds of surface and near-surface atoms. This can give rise to unusual surface phenomena and reactions.
The formation of nanoparticles from atoms involves two processes, namely, the formation of metal nuclei of different sizes and the interactions between the formed particles, which generate the formation of assemblies that possess a nanostructure.
Virtually all methods of nanosynthesis produce nanoparticles in nonequilibrium metastable states. On the one hand, this factor complicates their investigation and application in nanotechnologies aimed at the development of stable devices. On the other, nonequilibrium systems allow carrying out new unusual chemical reactions, which are difficult to predict.
Elucidation of the relationship between the size and chemical reactivity of a particle is among the most important problems of nanochemistry. For nanoparticles, two types of size effects are distinguished.27 One of these is their intrinsic or internal effect, which is associated with specific changes in superficial, bulk, and chemical properties of a particle. The other, external effect, represents a size-dependent response to external factors unrelated to the internal effect.
Specific size effects manifest themselves to a great extent for smaller particles and are most likely in nanochemistry, where irregular size–properties dependencies prevail. The dependence of activity on the size of the particles taking part in a reaction can be associated with the changes in the particle properties in the course of its interaction with an adsorbed reagent,28 correlations between geometrical and electron shell structures,29 and symmetry of boundary orbitals of a metal particle with respect to adsorbed-molecule orbitals.30
As mentioned above, nanochemistry studies the synthesis and chemical properties of particles and formations with sizes below 10 nm along one direction at least. Moreover, most interesting transformations are associated with the region of ca. 1 nm. Elucidation of mechanisms that govern the activity of particles with sizes of 1 nm and smaller is among the major problems of modern nanochemistry, despite the fact that the number of particles is a more fundamental quantity as compared with their size.
The dependence of chemical activity on the size of reacting particles is explained by the fact that properties of individual atoms of elements as well as of clusters and nanoparticles formed from atoms differ from the properties of corresponding macroparticles. To understand and roughly analyze the size-dependent chemical properties, we can compare the reactivities of compact substances, nanoparticles, and clusters of species.31 The demarcation lines between sizes of such formations vary from element to element and should be specified for each case.
In nanochemistry, the interaction of every particle with the environment has its own specifics. When studying individual properties of such a particle, attention should be focused on qualitative changes in particle properties as a function of its size. Moreover, the properties of isolated nanoparticles are characterized by a wide statistical scatter, which varies in time and requires special studies.
The internal size effect in chemistry can be caused by the changes in the particle structure and the surface-induced increase in the electron localization. Surface properties affect the stabilization of particles and their reactivity. For small numbers of reagent atoms adsorbed on the surface, a chemical reaction cannot be considered as in infinite volume, due to the commensurable surfaces of a nanoparticle and a reactant.
Reaction kinetics in small-scale systems with limited geometry differs from classical kinetics, because the latter ignores fluctuations in concentrations of reacting particles. Formations containing small numbers of interacting molecules are characterized by relatively wide fluctuations in the number of reactants. This factor gives rise to a time lag between the changes in reactant concentration on the surfaces of different-size nanoparticles and, as a consequence, to their different reactivities. Kinetics of such systems is described based on a stochastic approach,32 which takes into account statistical fluctuations in the number of reacting particles. The Monte-Carlo technique was also used for describing the kinetics of processes that occur on the surface of nanoparticles.33
In nanoparticles, a considerable number of atoms pertain to the surface, and their ratio increases with a decrease in the particle size. Correspondingly, the contribution of surface atoms to the system’s energy increases. This has certain thermodynamic consequences, for example, a size dependence of the melting point, Tm, of nanoparticles. The ...