Research is driven by the interplay of theory and experiment. Experiments give rise to theories, which we test using experiments, which drive theory, until we find answers to questions that interest us. After all, research is first and foremost about satisfying scientific curiosity.
Some experiments are easy to run. Most are not. Experiments require planning, and for most biological questions, expensive equipment, laboratory space, and abundant resources. Even more importantly, investigating any biological system means trying to unravel the interplay of numerous factors, the influence of which on the final outcome is not always easy to tease out. That is where mathematical modeling can come in very handy.
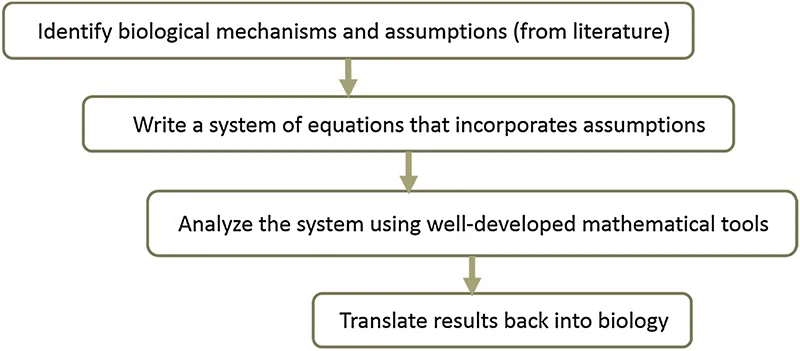
The schematic approach for using mathematical modeling for testing hypotheses about biological mechanisms can be summarized as follows:
In a way, mathematical modeling is like Rosetta Stone, a way to translate back and forth between mathematics and biology. As modelers, we try to (1) understand a biological mechanism, (2) tease out key players that drive the dynamics, (3) formulate assumptions about how these key players interact with each other and their environment, (4) translate these assumptions into equations, (5) analyze them using a very well-developed mathematical apparatus, and (6) translate the results back into the language of biology. If our predictions are consistent with experimental observations, then maybe we got it right, and we can now try to make further predictions about what will happen if we change this or that aspect of the system. Conducting early in silico experiments can save thousands of dollars when planning further experiments, advancing a “fiscally responsible” way of conducting research.
And what if the predictions made by the model are not consistent with experimental observations? That may be even more interesting. This means that some of our assumptions are incorrect, and thus our understanding of biology is incomplete. The good news is that since we built the model, we control all the assumptions, and so we can go through them one by one and identify which one is causing the discrepancy. Chances are we just identified a gap in knowledge, which we can now try to close using both theory and experiment.
However, all of this matters only when there is a question. A question that drives your curiosity, a question that bugs you like a pebble in your shoe. The question driving the work that led to this book stems from the following observation: sometimes after removal of primary tumors, metastases start growing faster. This has been observed particularly in colorectal cancer but in other cancer types as well. And the question is Why? What is going on?
It is possible that removing a primary tumor can result in some kind of malignant transformation, causing secondary tumors to form and rapidly start growing, but it is not very likely. Although some aggressive tumor types do exist, tumors typically take years and sometimes decades to form and grow. It is more likely that secondary tumors were there all along, either kept at bay or “sleeping,” and something either about the process of primary tumor removal, such as surgery, or the changes that happen in the body after the removal of the primary tumor “woke them up.” Cancers that are present but do not grow are known as dormant tumors, cancer without disease. Trying to understand mechanisms that underlie the state of dormancy might hold a key to answering the question underlying this book.
However, before we dive into that, it is important to gain some understanding about the basic mechanisms of mathematical modeling.
A Primer on Mathematical Modeling: Predator–Prey
A key aspect of understanding dynamical systems and writing mathematical models lies in thinking not of what things are but of what they do. This translates into thinking about relationships between individuals (people, animals, or cells), and how they interact with each other and their environment.
For instance, predator–prey type models have been developed extensively in the literature, starting from the classic Lotka–Volterra models that describe the dynamics of two populations, where the growth of one is dependent on consuming the other. These models were used to explain the dynamics of natural populations of lynx and snowshoe hare in Canada, for instance. However, predator–prey type models are also the classic framework for describing tumor–immune interactions, where tumor is the “prey” and immune system is “predator.” We will use this framework as an example for describing how to write a system of ordinary differential equations, which will be used in later chapters.
Equations describing tumor growth typically have the following components:
- • Growth terms, often logistic or “Gompertz,” of which logistic form is a special case
- • The carrying capacity (maximum population size that can be supported indefinitely) may remain fixed or can be dynamic, depending on the type of question the authors may be asking
- Death terms:
- • Death due to apoptosis or preprogrammed natural death (this can be included in logistic growth or as a separate term)
- • Death due to predation by the immune system
- • Death due to the activity of a drug or chemical agent
Equations describing the dynamics of the immune cells typically focus on CD8+T cells (white blood cells that have originated in the thymus and that express the CD8 proteins on cell surface) as the most efficient cytotoxic predators; however, they can be expanded to include antigen presenting cells (APCs), natural killer (NK) cells, etc., depending on the questions and the level of detail that is necessary to answer them. Notably, increasing the level of detail is not necessarily beneficial and can make it more difficult to discern the effects of the processes investigated. Therefore, the level of detail should be driven by the question, and only cell types and agents that quantitatively affect the dynamics, directly or indirectly, should be taken into account.
Equations describing the dynamics of immune cells typically have the following components:
- Growth terms:
- • Natural inflow (population growth) rates to describe baseline levels of the immune cells.
- • Additional inflow of immune cells due to the presence of the tumor. Since the immune cells react to the antigens/debris of the previously killed tumor cells, this term is proportional to the number of previously killed tumor cells. This is an important difference of tumor–immune models as compared to classic predator–prey type models, where increase of the predator would be directly proportional to how much prey it has consumed, as opposed to how many “carcasses” the predator has encountered.
- • In some variations, there may be other factors contributing to the ability of the predator (immune system) to grow. For instance, in Chapter 3 on tumor–immune interactions, we looked at the situation where both tumor and immune cells compete for glucose, a shared resource that is necessary for both populations to grow. Therefore, in this model, the term describing immune cell growth was also dependent on the level of available glucose.
- Death terms:
- • There often exists a natural death term for the tumor-specific immune cells to indicate decrease in immune cell levels following decrease in tumor levels.
- • There can also be a treatment-induced death term, which accounts for immune cell sensitivity to many cytotoxic drugs.
Once written, a system of differential equations is coded into a numerical solver, such as Matlab, and investigated numerically (in Matlab it is typically done using functions ode45 or ode23). To conduct a numerical investigation of a dynamical system, we identify parameters of interest for the question (this can be done based on understanding of the biology or through conducting sensitivity analysis, which will be discussed in later chapters), vary them, and create plots of how predicted population sizes change over time. This approach provides insights into what processes may be driving the dynamics of the investigated system, how different components of the system affect each other, and what may or may not be reasonable to try and reproduce in an experimental setting. Numerous examples will be given later in the book showing how this process is done.
Estimating Parameters
Many models appear to be overparameterized. As a rule, there should be as many parameters as are necessary for the units to balance out on both sides of the equation. That is, if we are tracking rate of change of population size over time on the left-hand side of the equation, we might have to introduce scaling parameters in the right-hand side terms to ensure balanced units.
Estimating parameter values for simulations may prove challenging because it may not always be possible to find experiments that will allow discerning exact values. Furthermore, in experiments, we may find that several parameters are combined into one, and discerning individual values for each may not always be possible or useful.
However, even in the absence of exact parameter values, it is important to be able to estimate the order of magnitude of the parameter value due to potential differences in time scales. If one of the described processes occurs qualitatively faster or slower than others, then failing to take this into account would attribute importance to a process that may not actually be affecting the dynamics, thus leading to incorrect or misleading predictions.
If one has access to experimental data, it may be possible to find parameters that allow reproducing the data using parameter fitting approaches such as least squares, Monte Carlo simulations, or built-in Matlab functions such as fmincon and fminsearch. This is also one of the best ways to validate the model before proceeding with further investigati...