- 224 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Organic Chemistry Concepts and Applications for Medicinal Chemistry
About this book
Organic Chemistry Concepts and Applications for Medicinal Chemistry provides a valuable refresher for understanding the relationship between chemical bonding and those molecular properties that help to determine medicinal activity. This book explores the basic aspects of structural organic chemistry without going into the various classes of reactions. Two medicinal chemistry concepts are also introduced: partition coefficients and the nomenclature of cyclic and polycyclic ring systems that comprise a large number of drug molecules. Given the systematic name of a drug, the reader is guided through the process of drawing an accurate chemical structure. By emphasizing the relationship between structure and properties, this book gives readers the connections to more fully comprehend, retain, apply, and build upon their organic chemistry background in further chemistry study, practice, and exams.- Focused approach to review those organic chemistry concepts that are most important for medicinal chemistry practice and understanding- Accessible content to refresh the reader's knowledge of bonding, structure, functional groups, stereochemistry, and more- Appropriate level of coverage for students in organic chemistry, medicinal chemistry, and related areas; individuals seeking content review for graduate and medical courses and exams; pharmaceutical patent attorneys; and chemists and scientists requiring a review of pertinent material
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Bonding in Organic Compounds
Abstract
Keywords
π-bond; σ-bond; Aromatic; Conjugated system; Electronegativity; Hybrid orbital; Orbitals; Resonance; Valence-shell electron-pair repulsionAtomic Orbitals
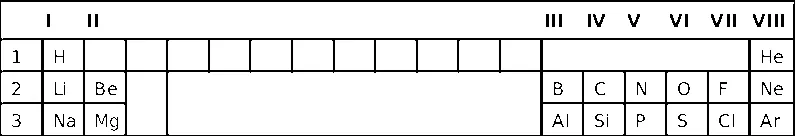
| I | II | III | IV | V | VI | VII | VIII | |||||||||||
| 1 | H | He | ||||||||||||||||
| 2 | Li | Be | B | C | N | O | F | Ne | ||||||||||
| 3 | Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Dedication
- Preface
- Chapter 1. Bonding in Organic Compounds
- Chapter 2. The Three-Dimensional Structure of Organic Compounds
- Chapter 3. Functional Groups
- Chapter 4. Acids and Bases
- Chapter 5. Partition Coefficients
- Chapter 6. The Nomenclature of Cyclic and Polycyclic Compounds
- Chapter 7. Drug Metabolism
- Index