
- 620 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About this book
Brazing processes offer enhanced control, adaptability and cost-efficiency in the joining of materials. Unsurprisingly, this has lead to great interest and investment in the area. Drawing on important research in the field, Advances in brazing provides a clear guide to the principles, materials, methods and key applications of brazing.Part one introduces the fundamentals of brazing, including molten metal wetting processes, strength and margins of safety of brazed joints, and modeling of associated physical phenomena. Part two goes on to consider specific materials, such as super alloys, filler metals for high temperature brazing, diamonds and cubic boron nitride, and varied ceramics and intermetallics. The brazing of carbon-carbon (C/C) composites to metals is also explored before applications of brazing and brazed materials are discussed in part three. Brazing of cutting materials, use of coating techniques, and metal-nonmetal brazing for electrical, packaging and structural applications are reviewed, along with fluxless brazing, the use of glasses and glass ceramics for high temperature applications and nickel-based filler metals for components in contact with drinking water.With its distinguished editor and international team of expert contributors, Advances in brazing is a technical guide for any professionals requiring an understanding of brazing processes, and offers a deeper understanding of the subject to researchers and engineers within the field of joining.- Reviews the advances of brazing processes in joining materials- Discusses the fundamentals of brazing and considers specific materials, including super alloys, filler metals, ceramics and intermetallics- Brazing of cutting materials and structural applications are also discussed
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
The wetting process in brazing
Abstract:
1.1 Introduction
1.2 Wetting of solids by liquid metals and oxides
1.2.1 Non-reactive wetting
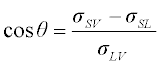
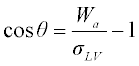
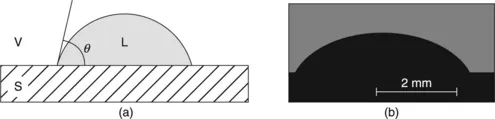
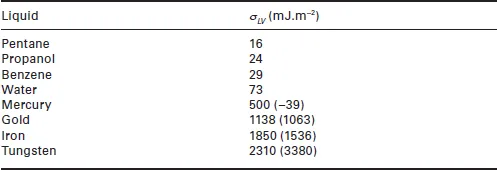
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Contributor contact details
- Preface
- Part I: Fundamentals of brazing
- Part II: Materials used in brazing
- Part III: Applications of brazing and brazed materials
- Index