
eBook - ePub
Exercise, Sport, and Bioanalytical Chemistry
Principles and Practice
- 136 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Exercise, Sport, and Bioanalytical Chemistry
Principles and Practice
About this book
A new volume in the Emerging Issues in Analytical Chemistry series, Exercise, Sport, and Bioanalytical Chemistry: Principles and Practice focuses on the basic and applied aspects of energy metabolism in humans. Concise and scientific, yet intelligible to the nonscientist, the book consists of two parts. Part I, Introduction: Basics and Background, provides the biochemistry necessary to understand the rest of the book and describes analytical processes and results as an aid to grasping the science. Part II, Applications: Knowledge into Practice, explores measurement techniques for metabolism, energy expenditure of various activities, techniques that enhance expenditure, metabolic adaptation, foods and drugs that enhance expenditure, and the role of bioanalytical chemistry in future research in exercise and sport. Discussion of the benefits of exercise and practices for improving the capacity to perform exercise is illustrated by many useful and entertaining examples. This volume allows readers to come away with a grasp of the scientific concepts, how they are manifested in research techniques, and how the results of research can be applied in the real world of public health and personal development.
The Emerging Issues in Analytical Chemistry series is published in partnership with RTI International and edited by Brian F. Thomas. Please be sure to check out our other featured volumes:
- Thomas, Brian F. and ElSohly, Mahmoud. The Analytical Chemistry of Cannabis: Quality Assessment, Assurance, and Regulation of Medicinal Marijuana and Cannabinoid Preparations, 9780128046463, December 2015.
- Tanna, Sangeeta and Lawson, Graham. Analytical Chemistry for Assessing Medication Adherence, 9780128054635, April 2016.
- Rao, Vikram, Knight, Rob, and Stoner, Brian. Sustainable Shale Oil and Gas: Analytical Chemistry, Biochemistry, and Geochemistry Methods, 9780128103890, forthcoming September 2016.
- Farsalinos, Konstantinos, et al. Analytical Assessment of e-Cigarettes: From Contents to Chemical and Particle Exposure Profiles, 9780128112410, forthcoming November 2016.
- Provides readers with the fundamental biochemistry and some elements of the physiology behind physical activity/exercise and describes the analytical techniques used to elucidate the science
- Written in clear, concise, compelling prose that is neither simplistic to scientists nor too sophisticated for a large, diverse global audience
- A one-page Close-Up in each chapter illustrates key topics to catch, engage, entertain, and create a novel synthesis of thought
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Exercise, Sport, and Bioanalytical Chemistry by Anthony C. Hackney,Anthony C Hackney in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Public Health, Administration & Care. We have over one million books available in our catalogue for you to explore.
Information
Part I
Introduction: Basics and Background
Outline
Chapter 1
Energy and Energy Metabolism
Abstract
Energy is essential for life, and the type of energy required is very specific. The body is designed to take chemical energy from food and convert it to the useful form, adenosine triphosphate (ATP). This conversion is critical for the performance of exercise. This chapter presents an overview of the principles of energy interconversion and how the body uses this principle to enable it to expend energy and perform the muscular work called exercise. Units of measurement are specified, anaerobic and aerobic pathways of ATP production and use are described, and a one-page “close-up” on the historical progression of methods for sampling and studying ATP processes is presented.
Keywords
Energy; adenosine triphosphate (ATP); work; energetics; exercise; anaerobic; aerobic; energy continuum
The aim of this chapter is to provide an overview of energy forms and types, and how chemical energy formation through biochemical reactions is essential for physiological functions in the healthy human body at rest and during the active state of exercise.
Energy
In physics, energy is a property that can be transferred between objects or states. The ability of a system to perform work is a more biological definition of energy and a meaning that can be applied to humans. Work in the biochemical and physiological sense is referred to as the energy transferred by mechanical means, or simply force applied or acting over a distance. During exercise, muscle does the work; that is, it applies a force (which requires energy) over a selected movement, distance, and pattern.1
Energy Transformation
The first law of thermodynamics, also called the conservation of energy principle, states that energy can be neither created nor destroyed, but it can exist in different classification forms: chemical, thermal, nuclear, electromagnetic, electrical, and mechanical. The body uses the first law daily as it consumes food, a form of chemical energy (macronutrients; see Chapter 2, “Energy Metabolism of Macronutrients During Exercise”), and converts it to useful chemical energy in the form of adenosine triphosphate (ATP).2 ATP consists of a base substance, adenine, attached to a sugar, the carbohydrate ribose, which has three phosphate molecules attached by high energy bonds. Removal or breakage of these phosphate bonds provides the energy for bodily processes.
Chemical Energy of the Body
The body is so dependent on ATP that it is called the “energy currency.” All physiological processes require it. ATP dependency is especially true for skeletal muscle, which provides the movement during exercise. The biochemical reaction by which ATP delivers useful energy can be represented as follows:
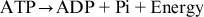
This reaction liberating energy involves the biochemical process of hydrolysis. ATP is broken down into adenosine diphosphate (ADP) by breakage of one of the high energy bonds and removal of a phosphate (Pi). The process is reversible; that is, rephosphorylation of ADP to ATP can occur by reattaching a Pi using the energy contained in food macronutrients when they are metabolized in select biochemical pathways.3 These ATP synthesis pathways are explained in more detail later but are simplistically categorized biochemically as either anaerobic (not requiring oxygen) or aerobic (requiring oxygen). Many exercise activities rely predominantly on one pathway. All-out explosive muscular movements such as sprinting 100 meters (m) as hard and fast as possible is primarily anaerobic and requires provision of ATP rapidly over a short period. A 20-kilometer (km;1 km=0.62 mile) run is predominantly aerobic; the muscular movement is not nearly as rapid, hence ATP can be produced more slowly, but large quantities are needed.4,5
Enzymes
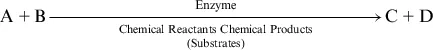
Biochemical reactions are catalyzed—that is, regulated—by proteins called enzymes. Enzymes influence the speed of a reaction, affecting the energy of activation, but they do nothing to alter the outcome. In the simple reaction formula below, the formation of the chemical products C and D could be occurring 100 million times quicker in the presence of an enzyme.6
The ATP reaction above is regulated in skeletal muscles as follows:
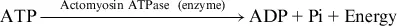
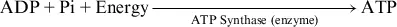
At the cellular level, signaling agents such as hormones can affect the activity rate of enzymes and in turn influence the speed at which reactions such as hydrolysis and rephosphorylation proceed (see Chapter 3, “Regulation of Energy Metabolism During Exercise”). These agents are among the major ways in which physiological events occurring at the cellular level are regulated.7
Energy Consumption
ATP is measured in moles (mol), but when researchers quantitate energy in humans it is typically done in kilocalories (kcal) per mol in the United States or kilojoules (kJ) per mol in Europe. The energy released by 1 mol of ATP is approximately 7.3 kcal or 30.5 kJ.8 The kcal (sometimes called a Calorie in the United States) is actually a thermal unit of energy developed over a century ago and represents the amount of heat energy necessary to raise the temperature of 1 kilogram (kg) of water 1°C. It can be used to express the amount of chemical energy contained in food items as well as energy liberated when exercise is performed (first law of thermodynamics).
Energy Transformation in Exercise
The average adult human (male 70 kg, female 62 kg) expends about 1 kcal/min (males slightly more, females slightly less) in a resting state. This is the resting metabolic rate (RMR), the amount of energy necessary each day to “just exist.” The RMR term is sometimes used interchangeably with basal metabolic rate (BMR), but the two are not exa...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Dedication
- Foreword
- Preface
- Acknowledgments
- Part I: Introduction: Basics and Background
- Part II: Applications: Knowledge into Practice
- Index