1.1 Introduction
Carrier-based drug delivery systems have attracted increased scientific interest for enhancing the efficacy of current drugs and particularly those with lower aqueous solubility. Among them, great interest has been developed to investigate carriers in the nanosize range to manufacture “nanomedicines” (Bang et al., 2009; Liu and Park, 2009; Xia et al., 2009). Nanocarriers are known to improve bioavailability, minimize degradation rate, control the release rate, reduce adverse effects, and enhance accumulation of the encapsulated drugs in the diseased target site (Torchilin, 2007). Controlled and sustained release of the active drugs from dosage forms is known to improve patient compliance toward the proposed treatment hence improving clinical outcomes (Shi et al., 2010).
Research in the area of nanoparticles started in the early 1960s with the introduction of parenteral emulsions, which helped in the administration of many low water soluble or lipophilic therapeutic agents. These parenteral emulsions were preferred as the method offered the advantages to be carried out on industrial scale (Kathe et al., 2014). However, some serious issues were associated with these emulsions like separation of the drug from the lipid phase into the aqueous phase was an unavoidable drawback. Similarly the storage or shelf life physical stability of emulsion systems was very inferior. Agglomeration followed by phase separation was evident in nearly all studies. The most striking problems associated with these emulsions were the achieving of a desired sustained release profile. Only extremely lipophilic drugs showed the desired release pattern (Washington, 1996; Prankerd and Stella, 1990).
To solve the problems associated with parenteral emulsions and other drug delivery systems, polymeric nanoparticles were subsequently introduced and developed. Polymer-based nanoparticles are advantageous in terms of their biocompatibility and biodegradability. Chemically modified and naturally occurring polymers are used to impart various functional characteristics to the nanoparticles. Though polymer-based nanoparticles are quite advantageous, still they have certain drawbacks like toxicity, long residence time, residual organic solvents, and industrial scale-up of the process. Liposomes were developed as alternative to overcome the above-cited drawbacks. They were quite biocompatible and biodegradable and had the advantage to deliver many potent drugs that otherwise have serious side effects. Moreover, hydrophilic drugs were successfully entrapped in the aqueous compartments of the liposomal vesicles. This drug delivery system was found to have some inherent shortcomings of low physical stability, nonspecificity, drug expulsion, and clearance by macrophages (Samad et al., 2007; Couvreur et al., 1995).
In the early 1990s, researchers focused their attention toward the development of nanoparticles based on lipid matrices that would be solid at room temperature. This system was intended for drug dosage form based on inert lipids having a solid matrix and that would be quite enough in limiting the drug mobility and providing increased stability. This gifted drug delivery system acquired the advantages of polymeric nanoparticles and micronized emulsions and is known as solid lipid particles (SLNs) (Soppimath et al., 2001; Smith, 1986). By definition, they are colloidal particles in submicron (50–1000 nm) level, consist of biocompatible and biodegradable solid lipids (lipids that are solid at room temperature), stabilized with surfactants, polymers, or their mixtures, and capable to host both lipophilic and hydrophilic drugs. SLNs have emerged as promising drug delivery system bearing the treats, functionalities, and advantages of different carrier systems (Harde et al., 2011; Gasco, 1993). Due their simplicity, versatility, and being promising drug carriers, SLNs have greatly attracted the attention of scientific community involved in formulation. Owing to their definition, they are small size colloidal particles, and are considered to be important from many aspects. As the smaller the particle size, the more likely they are to remain stable, the more potential for targeted responses, and the more capacity to encapsulate increased amounts of drugs (Müller et al., 2002; Wissing et al., 2004). They are a new generation of lipid-based emulsions in submicron-sized where the liquid lipid (oil) has been replaced with a solid lipid. They possess unique properties like small size, high drug loading capacity, large surface area, and the interaction of phases at the interfaces. They have been attractive for their potential to improve the therapeutic efficacy of pharmaceuticals, nutraceuticals, and other materials (Cavalli et al., 1993). Being similar to polymeric nanoparticles, their solid matrix provides great protection to the loaded active ingredients against chemical degradation under harsh biological environment. It also helps the modulation of the drug release profiles. Furthermore, they can be synthesized at mega industrial scale through high-pressure homogenization. All these constructive attributes make SLNs excellent carriers for drug delivery (Harde et al., 2011).
SLNs research has gained wide global importance recently as large number of drugs are formulated using this technique. SLNs are commonly used (1) for parenteral delivery of drugs (Yang et al., 1999), (2) to enhance the oral bioavailability of lipophilic drugs that are not manageable with other delivery systems (Abuasal et al., 2012; Hu et al., 2004), (3) for ocular drug delivery in order to improve their corneal penetration and residence time in the eye (Seyfoddin et al., 2010), (4) for topical drug delivery to treat different skin diseases (Schäfer-Korting et al., 2007), and (5) for pulmonary and rectal drug delivery (Liu et al., 2008; Sznitowska et al., 2001). Targeting delivery of drugs to specific diseased sites has also been reported by different researchers (Chattopadhyay et al., 2008).
1.3 Structural Composition of Solid Lipid Nanoparticles
Structurally, SLNs are composed of solid lipid(s), surfactant(s), cosurfactant (if needed), and active pharmaceutical ingredients (drugs). All the lipids used in the production of SLNs are of physiological nature having broad structural diversity. The lipids used in the production are broadly categorized into fatty alcohols, fatty acids, fatty esters, partial glycerides or triglycerides. Few research groups have also reported waxes to be used in the production of these nanoparticles (Jenning and Gohla, 2000). SLNs are surface-tailored with surfactants, thus resulting in the enhanced stability of the colloidal system. They are sometimes used in combination with a cosurfactant, if necessary. All the structural components of SLNs are discussed one by one in detail.
1.3.1 Lipids
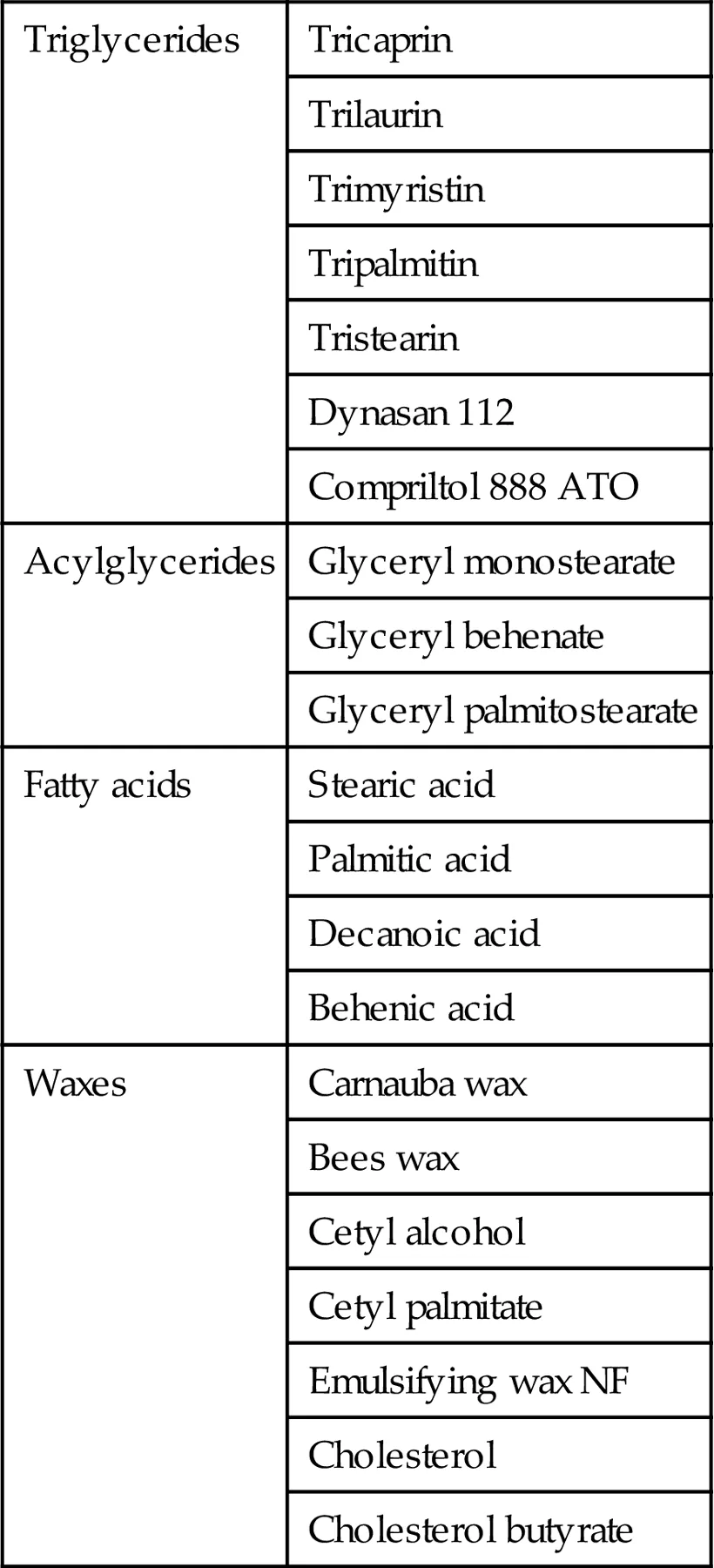
Being the major constituents of SLNs, solid lipids are considered to be responsible for the stability, release, the entrapment and drug loading. Ideally, they are the lipids which dissolved the drugs in them. Few of the lipids that are frequently employed in SLNs production are fatty acids, steroids, waxes, triglycerides, acylglycerols and their combinations as shown in Table 1.1. Most of the lipids, except that of cetyl palmitate, have been approved as generally-recognized-as-safe. They all are compatible and physiologically well tolerated (Mehnert and Mäder, 2001).
Table 1.1
Solid Lipids Used in Preparation of Solid Lipid Nanoparticles
| Triglycerides | Tricaprin |
| Trilaurin |
| Trimyristin |
| Tripalmitin |
| Tristearin |
| Dynasan 112 |
| Compriltol 888 ATO |
| Acylglycerides | Glyceryl monostearate |
| Glyceryl behenate |
| Glyceryl palmitostearate |
| Fatty acids | Stearic acid |
| Palmitic acid |
| Decanoic acid |
| Behenic acid |
| Waxes | Carnauba wax |
| Bees wax |
| Cetyl alcohol |
| Cetyl palmitate |
| Emulsifying wax NF |
| Cholesterol |
| Cholesterol butyrate |
Prior to their use in the production of SLNs, selection of suitable lipids is an important parameter so to predict the essential characteristics of the nanoparticles. Though no solid guidelines are available, empirical values, such as the solubility of drug in the lipid have been suggested as suitable criteria for selection of a suitable lipid (Bummer, 2004). The solubility of drugs in lipid matrices is critical because it greatly influences the drug entrapment efficiency and loading potential, consequently decides the effectiveness of the lipid nanoparticles as drug delivery system (Kasongo et al., 2011). Using UV-Visible spectroscopy or other chromatographic techniques, the drug solubility can be easily investigated. The drug partitioning between the lipid/oil and aqueous phases can also be assumed following mathematical approaches. These predictions are based on interactions of drug–lipid and drug–water. SLNs can be prepared with increased drug loading capacity if the drug is highly soluble in lipid or having high partition coefficient. As a drug has different solubility in different lipids, its apparent partition coefficients differ for different lipids. This consequently leads to different loading potential in different lipid matrices for the same drug. Though these methods are helpful in selecting a lipid for formulations, their complications are still hindering the prediction of highly compatible and suitable lipids with desirable properties (Shah et al., 2015b).
The type and structure of the lipid used greatly affect SLNs characteristics like size of the particles, stability, drug encapsulation efficiency, and release profile. Generally, it has been noted that average particle size of SLNs dispersion increases when higher melting lipids are used. The main technical point behind this phenomenon is higher viscosity of dispersed phase. Some parameters are specific for every lipid like lipid crystallization, shape of lipid crystals, and lipid hydrophilicity. Most of the lipids are mixtures of different compounds; as a result their composition can be different when obtained from different suppliers. There can also be batch to batch variations. These variations affect the quality of SLNs to a great extent; and can retard crystallization processes, changing the zeta potential and much more like these. When lipid contents in SLNs formulations are increased over 5%–10%, this mostly leads to larger particles and broader particle size distribution (Mehnert and Mäder, 2001; Müller et al., 2002; Chakraborty et al., 2009). As a general practice, lipids with increased lipophilicity results in the increased amount of the hydrop...