
- 178 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Essential Pharmacokinetics: A Primer for Pharmaceutical Scientists is an introduction to the concepts of pharmacokinetics intended for graduate students and new researchers working in the pharmaceutical sciences. This book describes the mathematics used in the mammillary model as well as the application of pharmacokinetics to pharmaceutical product development, and is useful as both a self-study and classroom resource. Content coverage includes detailed discussions of common models and important pharmacokinetic concepts such as biological half-life, clearance, excretion, multiple dosage regimens and more. Numerous equations, practical examples and figures are incorporated to clearly illustrate the theoretical background of pharmacokinetic behavior of drugs and excipients.
- Shows how to apply basic pharmacokinetic methods to evaluate drugs, excipients and drug products
- Uses guided practice questions, mathematical concepts and real-world examples for self-assessment and retention purposes
- Illustrates how to write and evaluate drug registration files
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Essential Pharmacokinetics by Thorsteinn Loftsson in PDF and/or ePUB format, as well as other popular books in Medicine & Pharmacology. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
Introduction
Pharmacokinetics is a study of drug and metabolite kinetics in the body: drug absorption, distribution, metabolism, and excretion. Pharmacokinetic models and mathematic models are used to calculate drug dosage regimens, perform dosage adjustments in patients, predict food–drug and drug–drug interactions, design and test drug formulations and novel drug delivery systems, and evaluate the quality of pharmacologic products. The most common pharmacokinetic model is the mammillary model. It is an abstract model, in which one or more compartments represent the whole body or group of tissues and where the movement of drug molecules from one compartment to another, as well as drug excretion and metabolism, follows first-order kinetics.
Keywords
Compartmental models; duration; mammillary model; onset time; pharmacokinetic model; population pharmacokinetics; therapeutic index
Although the concept of drug absorption, distribution and elimination has been known for over 150 years [1] the term “pharmacokinetics” was first introduced in 1953 by Friedrich Hartmut Dost in his book “Der Blütspiege: Kinetic der Konzentrationsabläufe in der Krieslaufflüssigkeit” [2,3]. Later Perl [4], Nelson [5], Krüger-Thiemer [6], Wagner [7,8], Garrett [9,10], Rowland [11], Gibaldi [12,13], Riegelman [14], Levy [15], and numerous other scientists introduced the various pharmacokinetic methods and terms, giving us the science of pharmacokinetics as it is today [1].
1.1 Some Basic Concepts
A drug proceeds through a distinct pathway from mixing the active pharmaceutical ingredient (API) with excipients to forming the drug product to the therapeutic effect (Figure 1.1). For example, a propranolol tablet is formed by compressing a mixture of the API (i.e., propranolol hydrochloride) and various excipients such as lactose into a tablet. Tablets are one of several different propranolol drug products. Other known propranolol products include oral solutions and solutions for parenteral injection. Following oral administration (sometimes referred to as per os or per oral [PO] administration), the tablet disintegrates, and solid propranolol dissolves in the aqueous fluid of the gastrointestinal (GI) tract. The dissolved propranolol molecules are then absorbed into the general blood circulation and distributed throughout the body. The drug is partly metabolized and excreted from the body, but a small fraction of the drug, which is a β-blocker, reaches the target site, where its binds to receptors (e.g., β-adrenergic receptors), causing vasodilatation (which is the pharmacologic response) that leads to lowering of blood pressure (which is the therapeutic effect). Pharmacokinetics is the kinetics of drug absorption, distribution, metabolism, and excretion (ADME). All of these four criteria influence the levels and kinetics of drug exposure to tissues and thus influence the performance and pharmacologic activity of the compound as a drug. ADME profiling and toxicology screening are some of the most important research activities in the drug discovery and development process. ADME and toxicologic (ADME/Tox) properties determine the “druggability” of new chemical entities (NCEs). Biopharmaceutics describes how the physicochemical properties of drugs, the pharmaceutical dosage forms, and the routes of drug delivery affect the rate and extent of drug absorption into the body. Pharmacodynamics is the science that describes the relationship between the drug concentration at the receptor and biological activity (i.e., pharmacologic response or drug effect).

After oral administration, the drug is absorbed from the GI tract into the body (Figure 1.2). In general, some fraction of the drug is then metabolized and the metabolites excreted through urine, but a fraction of the drug may also be excreted unchanged through urine.

Bioavailability represents the drug fraction that reaches the systemic blood circulation after, for example, oral administration. Bioavailability can be divided into pharmaceutical availability and biologic availability. If propranolol is completely released from a tablet and dissolved in the aqueous GI fluid, the drug is said to have 100% pharmaceutical availability. Aqueous propranolol solution has 100% pharmaceutical availability (Fpharm). However, propranolol undergoes first-pass metabolism and thus its biologic availability (Fbio) after oral administration is frequently about 75%. Consequently, the bioavailability (F) of propranolol solution will be only about 75%:
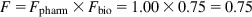
If the pharmaceutical availability of propranolol in a tablet is 50% and the biologic availability is 75%, the bioavailability of the propranolol tablets will be 37.5%:
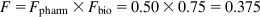
Drugs have 100% bioavailability when they are administered through intravenous (IV) injection, that is, the entire drug dose enters the general blood circulation. Minimum effective concentration (MEC) is th...
Table of contents
- Cover image
- Title page
- Table of Contents
- Dedication
- Copyright
- Preface
- Chapter 1. Introduction
- Chapter 2. Basic Concepts of Pharmacokinetics
- Chapter 3. Physicochemical Properties and Pharmacokinetics
- Chapter 4. Drug Pharmacokinetics After Alternative Routes of Administration
- Chapter 5. Pharmacologic Response and Drug Dosage Adjustments
- Chapter 6. The Effect of Food and Excipients on Drug Pharmacokinetics
- Chapter 7. Practice Problems
- Appendix I. Answers to Problems in Chapter 7
- Appendix II. Symbols and Abbreviations
- Index