
eBook - ePub
Transition Metal-Catalyzed Pyridine Synthesis
Transition Metal-Catalyzed Heterocycle Synthesis Series
- 90 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Transition Metal-Catalyzed Pyridine Synthesis
Transition Metal-Catalyzed Heterocycle Synthesis Series
About this book
Transition Metal-Catalyzed Pyridine Synthesis provides an overview of pyridines, describing properties of these heterocycle compounds and describing traditional synthetic procedures for them. The book then explores catalyzed procedures for pyridine synthesis in greater detail and depth than is currently available in published Review articles.
The short series Transition Metal-Catalyzed Heterocycles Synthesis, authored by Xiao-Feng Wu, summarizes recent achievements on heterocycles synthesis with transition metal as the catalysts, with each volume dedicated to one heterocycle compound.
- Brief, focused review of this active research area, Pyridine synthesis via transition metal catalysis
- Useful coverage of Pyridine properties and both intermolecular and intramolecular cyclization
- Volume Two in Elsevier's short work series, "Transition Metal-Catalyzed Heterocycles Synthesis"
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Transition Metal-Catalyzed Pyridine Synthesis by Xiao-Feng Wu in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Organic Chemistry. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
Introduction
Abstract
A short introduction on pyridine derivative has been given.
Keywords
Pyridine; biological active; organic synthesis
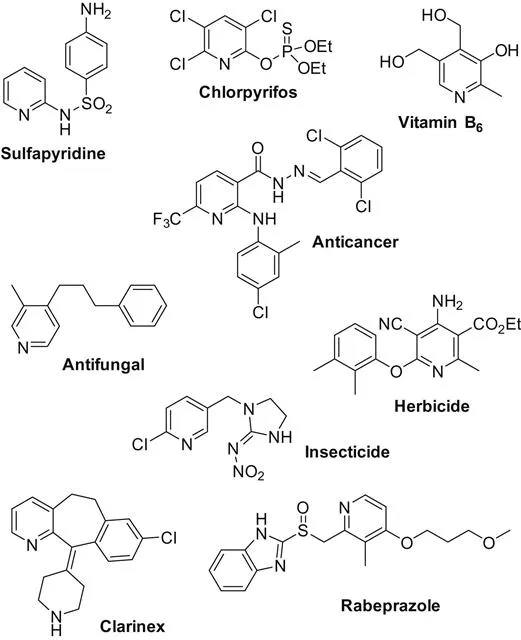
Pyridine is an important class of nitrogen-containing heterocycles found in various natural products, pharmaceuticals, and materials (Scheme 1.1). Based on its importance, numerous synthetic procedures have been developed for their preparation [1]. In this book volume, the main achievements on transition metal-catalyzed pyridines synthesis are discussed. Based on the reaction types, the whole volume is catalogued by intramolecular cyclization and intermolecular cyclization reactions.
Chapter 2
Synthesized by Intramolecular Cyclizations
Abstract
The procedures based on transition metal-catalyzed intramolecular cyclizations to prepare pyridines have been discussed here.
Keywords
Pyridine; intramolecular cyclization; organic synthesis; synthetic methodology; carbonylation; coupling
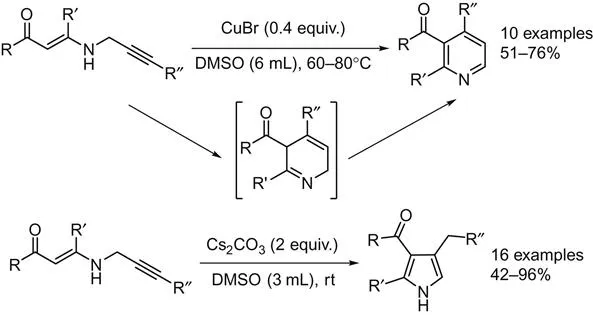
Intramolecular cyclization of substrates to the corresponding pyridine derivatives is the most straightforward pathway. By intramolecular version reaction, the positions of substituents are fixed and easier to control, such as N-propargylic β-enaminones in pyridines and pyrroles synthesis. In 2008, Cacchi et al. reported a copper-catalyzed synthesis of polysubstituted pyridines from N-propargylic β-enaminones [1]. By using DMSO as the solvent, pyrroles can be produced in good to high yields in the presence of only Cs2CO3 at room temperature, while pyridines can be formed under the assistant of CuBr at 60–80°C (Scheme 2.1). Here, the N-propargylic β-enaminones can be prepared via the following sequences: (1) cross-coupling of terminal alkynes with acyl chlorides; (2) followed by the conjugate addition of propargylamine with the resultant α,β-enones; (3) further Sonogashira cross-coupling of the propargyl derivative with aryl halides.
A procedure by using enamino ester and alkynone as the substrates was developed as well [2]. 2,3,4,6-Tetrasubstituted pyridines were prepared in a single step. Various acids, such as acetic acid, Amberlyst 15 ion exchange resin, zinc(II) bromide or ytterbium(III) triflate, can be applied as promoter for the cyclization step of the Michael addition adduct. 4-(3-Oxoalkyl)isoxazoles found could be applied as starting material for pyridine synthesis as well [3].
In 2013, Kim and coworkers reported a palladium-catalyzed domino cyclization of N-(2-bromoallyl)-N-cinnamyltosylamides for the construction of pyridines [4]. The reaction proceeds via a domino 5-exo/3-exo carbopalladation, ring expansion by palladium rearrangement, and an aromatization. Various 4-arylnicotinate derivatives were produced in good yields (Scheme 2.2).
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Dedication
- Chapter 1. Introduction
- Chapter 2. Synthesized by Intramolecular Cyclizations
- Chapter 3. Synthesized by Intermolecular Cyclizations
- Chapter 4. Summary and Outlook