
- 294 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Alkaloids are a large group of structurally complex natural products displaying a wide range of biological activities. The purpose of Alkaloids: A Treasury of Poisons and Medicines is to classify, for the first time, the alkaloids isolated from the natural sources until now. The book classifies all of the alkaloids by their biosynthetic origins. Of interest to the organic chemistry and medicinal chemistry communities involved in drug discovery and development, this book describes many alkaloids isolated from the medicinal plants, including those used in Japanese Kampo medicine.- Classifies and lists alkaloids from natural sources- Occurrence and biosynthetic pathways of alkaloids- Indicates key uses and bioactivity of alkaloids
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Alkaloids by Shinji Funayama,Geoffrey A. Cordell in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Organic Chemistry. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
Alkaloids Derived from Phenylalanine and Tyrosine
Abstract
This chapter introduces those alkaloids derived from phenylalanine and tyrosine.
Keywords
Aristolochic acid; Berberine; Chelidonine; Coclaurine; Colchicine; d-tubocurarine; Dopamine; Emetine; Galanthamine; L-DOPA; Lycorine; Morphine; Phenylethylamine; Thyroxine
The thousands of alkaloids derived from phenylalanine and tyrosine possess a wide range of important biological activities, and several of them are pharmaceutical agents, present in various traditional medicines in various systems, or serve as biological tools. In this chapter, those alkaloids are discussed, from the simplest alkaloids to those that represent more complex chemical structures. The coclaurine-type alkaloids are one of the simplest of these alkaloids, and are described in Section 1.4. Among them, reticuline, in its antipodal forms, is an important biosynthetic precursor of various alkaloids, including such alkaloids as berberine and morphine. Some of the alkaloids of this type are derived through highly complicated, and incompletely understood, biosynthetic pathways. For example, it is very difficult to elucidate the original amino acid derivations of colchicine (Section 1.11) and lycorine (Section 1.13) from a superficial examination of their chemical structures.
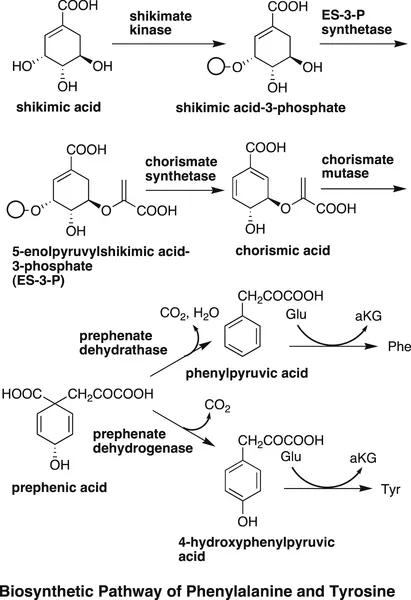
Both phenylalanine and tyrosine are derived from chorismic acid, which is itself derived from shikimic acid-3-phosphate through the shikimic acid pathway. In this sequence, chorismic acid is first transformed into prephenic acid by chorismate mutase. If prephenic acid is converted into phenylpyruvic acid by the action of prephenate dehydratase and a transaminase, phenylalanine is formed. On the other hand, if it is transformed into 4-hydroxyphenylpyruvic acid by the action of prephenate dehydrogenase, followed by a transaminase reaction, then tyrosine is formed.
1.1
Phenylethylamines (Phenethylamines)
Peyote (Lophophora williamsii, syn. Anhalonium williamisii) is a cactus and member of the family Cactaceae, and grows wild in the deserts of Mexico and the southern United States [1]. The cactus is also cultivated in Japan as a decorative plant and known as “Ubatama.”
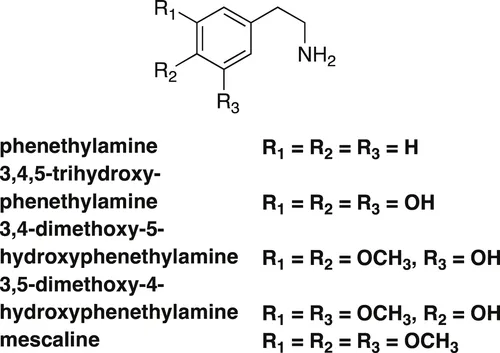
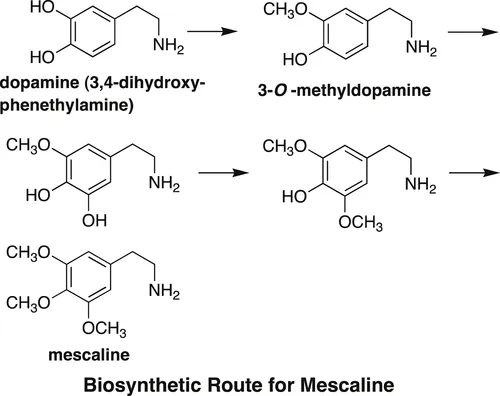
This cactus is an important source of the so-called phenylethylamine (phenethylamine) alkaloids with a C6C2N skeleton. The main component of the alkaloid mixture is mescaline. The name mescaline is derived from the name of the cactus, which is also known as “mescal buttons.” Mescaline is known to possess hallucinatory effects and a number of undesirable side effects. With respect to the biosynthesis of mescaline, it was shown that L-tyrosine is oxidized to give L-DOPA (L-3,4-dihydroxyphenylalanine), which is transformed to the biosynthetic precursor dopamine (3,4-dihydroxyphenethylamine) [2], which is selectively O-methylated to afford 3-O-methyldopamine, a key biosynthetic precursor of the alkaloid. The direct biosynthetic precursor of mescaline was determined to be 3,5-dimethoxy-4-hydroxyphenethylamine, because 3,4,5-trihydroxy phenethylamine and 3,4-dimethoxy-5-hydroxyphenethylamine were not incorporated into the biosynthetic pathway to mescaline [2].
Hordenine and N-methyltyramine are isolates from the young roots of Hordeum vulgare var. hexastichon (Poaceae), and are simple phenylethylamine-type alkaloids. The biosynthetic precursor of these alkaloids is considered to be tyramine, derived from tyrosine. dl-[2-14C]-Tyrosine was fed to H. vulgare var. hexastichon 4 days after germination, and hordenine and N-methyltyramine were isolated after 11 days from the roots. Both alkaloids possessed 14C label at the α-carbon. It was also found that dl-[2-14C]-tyrosine was more effectively incorporated into N-methyltyramine than into hordenine, and no tyramine was detected in the extract. So, the incorporated tyrosine was converted into tyramine and methylated immediately to give N-methyltyramine. Subsequent steps form hordenine by the methylation of N-methyltyramine [3].
On the other hand, it was clarified by the incorporation of labeled methionine that the methyl groups incorporated during the biosynthesis of N-methyltyramine and hordenine were derived from methionine. The yield of N-methyltyramine in these experiments is less than half that of hordenine; however, the incorporation of 14C into N-methyltyramine is 1.5 times that of hordenine. This indicates that N-methyltyramine is not formed by the demethylation of hordenine, but th...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Foreword
- Introduction
- Chapter 1. Alkaloids Derived from Phenylalanine and Tyrosine
- Chapter 2. Alkaloids Derived from Tryptophan
- Chapter 3. Alkaloids Derived from Ornithine and Arginine
- Chapter 4. Alkaloids Derived from Lysine
- Chapter 5. Alkaloids Derived from Proline
- Chapter 6. Alkaloids Derived from Glutamic Acid
- Chapter 7. Alkaloids Derived from Histidine
- Chapter 8. Alkaloids Derived from 2,3-Diaminopropionic Acid
- Chapter 9. Compounds Derived from Anthranilic Acid
- Chapter 10. Alkaloids Derived from Nicotinic Acid
- Chapter 11. Alkaloids Derived from Nucleic Acids and Related Compounds
- Chapter 12. Alkaloids Possessing the Porphine Skeleton
- Chapter 13. Alkaloids Derived from an m-C7N Unit
- Chapter 14. Alkaloids Derived from Terpenoids
- Chapter 15. Alkaloids Derived from Polyketides
- Chapter 16. Alkaloids Derived from a C6–C1 Unit
- Index