
- 496 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Given the infinite number of applications of polymeric materials in everyday life, especially applications where a failure in service may lead to economic loss, injury or death, the ability to determine the cause of failure using forensic engineering techniques is essential. Forensic polymer engineering: Why polymer products fail in service reviews the latest forensic engineering techniques used in the investigation of failed polymer materials. It presents a series of case studies which illustrate the different types of failure and the forensic engineering techniques used in their investigation.The first chapters give an introduction to forensic polymer engineering and an overview of the examination and analysis of failed polymer components. Further chapters give detailed case studies of failure and forensic investigation of polymeric medical devices, polymer storage tanks, small polymeric containers, polymer pipes and fittings, polymeric seals, polymeric tools and ladders, polymer components in transport applications and polymer consumer products. A final concluding chapter provides information on causes of product failure and discusses poor manufacturing methods, poor design, poor choice of materials and failure due to insufficient account being taken of environmental factors.With its distinguished authors, Forensic polymer engineering: Why polymer products fail in service is a standard reference for forensic experts practicing in all engineering fields that involve polymeric materials, as well as design and construction professionals, product manufacturers and insurance professionals.
- Reviews the latest forensic engineering techniques used in the investigation of failed polymer components
- Detailed case studies illustrate different types of failure in polymer components, fittings and medical devices
- Examines the role of manufacturing in product failure with an overview of faults recognised in methods, design and material selection
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Forensic Polymer Engineering by Peter Rhys Lewis in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Materials Science. We have over one million books available in our catalogue for you to explore.
Information
1
Introduction
1.1 Product failure
It comes as no surprise that products have a limited life in service. But what many might find surprising is the very great range of possible causes of failure, from a large and now very diverse range of materials. The failure modes of most metals are well established, simply because most have been used in service for many years. They have been well studied both in the laboratory and in practical applications, so there is a voluminous literature on the way they fracture, or fail in other ways. That, of course, does not stop further failures, but it does make failures from known causes less likely. Engineers and designers have a large property database available to them to check whether or not a particular metal or alloy is fit to be used under a specified set of circumstances. Such is not the case with most non-metallic materials, especially those that have been discovered or invented within the recent past, especially polymers. Their failure mechanisms are the subject of this book, but we have not taken the usual academic approach of separating the failure mechanism from the product which fails, but rather discuss each incident as a case study in its own right.
Case studies are important for several reasons. Product failures must be discussed in context, when the cause or causes of failure can be related to the way in which the product has been used (or abused). Secondly, if further failures of a particular type are to be prevented in future, then the causes must be identified so as to take remedial measures. It necessarily implies that all the product features which are relevant to its failure have to be examined for establishing the causal chain of events leading to its final demise. The first step in establishing the causal chain is simply to provide a chronology of events, so that each step is isolated and sequenced. Only then can the causes be tackled, using appropriate analytical tools. The details of each incident have to be described so that the critical facts can be sorted from the mass of irrelevant detail. This enables a fuller picture of the accident to be achieved, and it is also much more interesting to the reader if he or she wishes to draw parallels with related incidents within their own experience. There is no better way of illustrating the basic principles of polymer technology than by way of a detailed case study. It focuses attention on a specific aspect of the polymer structure, or the way it has been made, or the design of the product which has failed.
So by way of prelude to the case studies described in this book, it is essential to provide the technical backdrop to the diversity of polymers used in products today. They provide properties unavailable in metals, such as transparency, low weight, high strength and insulation, for example. Low weight is at a premium for transport of goods and people, and one area where polymers have expanded in use. That success forms the backdrop to this book. After all, to understand the way materials succeed helps to understand why they fail. The starting point is the range of non-metals available in the natural spectrum of elements. General definitions of terms and some explanatory text is provided in two dictionaries (1,2).
1.1.1 Non-metallic elements
The breadth of materials is not only very wide, but growing at an unprecedented rate today. To the existing fixed number of naturally occurring metals (about 72 of the 92 elements) have been added many different alloys, and compositions for particular kinds of properties. The much smaller number of non-metallic elements (about 20) exert an influence way beyond their number, which is actually only about 14, when the unreactive noble gases are excluded. That select group of elements includes most important of all, the elements: carbon (C), silicon (Si), oxygen (O), hydrogen (H), nitrogen (N), sulphur (S) and phosphorus (P). They are abundant in the Earth’s crust, and are all reactive both with one another, and with the metals.
With the large class of metals and alloys, they include polymers, ceramics and glasses, all of which are important classes of useful solid materials. But in order to understand their physical and chemical properties, a brief discussion of the way they are held together at a molecular and atomic level is useful. The bonding then gives rise to various kinds of structure, depending on whether or not the bonds are directional or otherwise.
1.1.2 Bonding
All solid materials are held together by bonds between the atoms of which they are made. The major classes of bond include:
• metallic
• covalent or chemical
• electrostatic
• hydrogen bonds
• van der Waals bonds.
The first three bond types are the strongest, the last two the weakest, and it is natural that the first group dominate the major classes of material. The covalent and hydrogen bonds are highly directional in space compared with the non-directional metallic, electrostatic and van der Waals bonds. They can therefore be symbolized by lines in flat representations, as in the following pages, and covalent bonds give rise to many important engineering materials, especially polymers and composites.
Covalent bonds
Excluding the metallic bond, covalent bonds occur when elements combine together and form a stable compound. The simplest example is the hydrogen molecule, written symbolically as H2, because it is a compound of two hydrogen atoms linked by a single covalent bond:

The bond is symbolised by the line between the two atoms, and hydrogen is said to be a diatomic molecule. It exists as a gas under normal conditions, is the lightest gas known and so has been used for lifting airships, for example. It is highly explosive in mixtures of air or oxygen, a problem encountered in a range of failing products. It occurs in a similar covalently bonded form in many compounds with carbon, such as in the thermoplastic polyethylene:
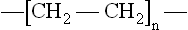
This representation is known as a repeat unit, because when repeated endlessly, it creates a very long chain molecule. The real material is thus made from a mixture of such long chains. Structural complexity occurs when further groups are added to the simple PE repeat unit, so polypropylene has a methyl group added:
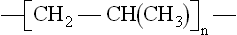
But hydrogen also occurs in more complex repeat units, not just with carbon but also with other elements such as nitrogen and oxygen, as in the thermoplastic material nylon 6, with repeat unit:
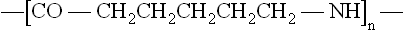
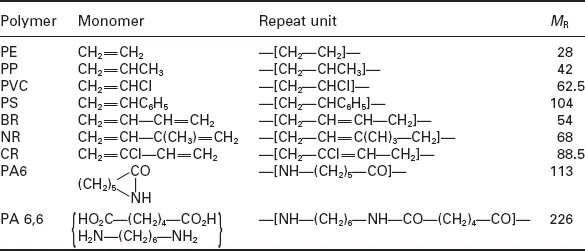
All polymers can be described by a repeat unit, or combination of different repeat units (copolymers), as shown for a few simple polymers in Table 1.1. The monomers from which they are made are also shown, together with the molecular weight of the repeat unit (MR). The latter can be calculated using standard atomic weights and knowing the repeat unit formula. Thus since the atomic weight (relative atomic mass) of carbon and hydrogen are 14 and 1 respectively, M is (2 × 12) + (4 × 1) = 28. Copolymer structure gives an added level of complexity, as shown in Fig. 1.1 for the various structures formed from styrene, butadiene and acrylonitrile monomers.
Table 1.1
Repeat units and size in some common polymers
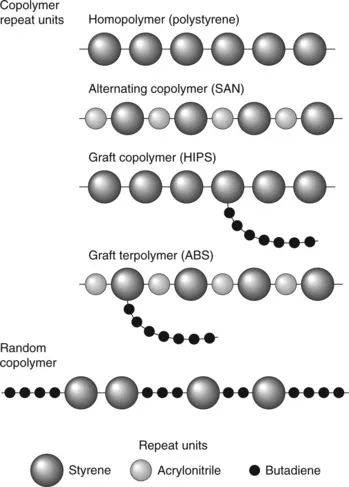
1.1 Co-polymer repeat units.
Polymers can also be classified as thermoplastic and thermoset, terms which describe their behaviour on heating. Thermoplastics can be heated repeatedly with little change in properties, while thermosets cross-link on heating. Cross-linking binds all the chain molecules together by covalent bonds, so that the shape of the material...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Preface
- Acknowledgements
- Dedication
- Chapter 1: Introduction
- Chapter 2: Examination and analysis of failed components
- Chapter 3: Polymeric medical devices
- Chapter 4: Polymer storage tanks
- Chapter 5: Small polymeric containers
- Chapter 6: Polymeric pipes and fittings
- Chapter 7: Polymeric seals
- Chapter 8: Tools and ladders
- Chapter 9: Components in transport applications
- Chapter 10: Consumer products
- Chapter 11: Conclusions
- Index