
Fuel Cells and Hydrogen
From Fundamentals to Applied Research
- 296 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About this book
Fuel Cells and Hydrogen: From Fundamentals to Applied Research provides an overview of the basic principles of fuel cell and hydrogen technology, which subsequently allows the reader to delve more deeply into applied research. In addition to covering the basic principles of fuel cells and hydrogen technologies, the book examines the principles and methods to develop and test fuel cells, the evaluation of the performance and lifetime of fuel cells and the concepts of hydrogen production.Fuel Cells and Hydrogen: From Fundamentals to Applied Research acts as an invaluable reference book for fuel cell developers and students, researchers in industry entering the area of fuel cells and lecturers teaching fuel cells and hydrogen technology.- Includes laboratory methods for fuel cell characterization and manufacture- Outlines approaches in modelling components, cells and stacks- Covers practical and theoretical methods for hydrogen production and storage
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Introduction
† Graz University of Technology, Institute for Chemistry and Technology of Materials, Graz, Austria
‡ Graz University of Technology, Institute of Chemical Engineering and Environmental Technology, Graz, Austria
Abstract
Keywords
1.1 Electrochemical Systems and Fuel Cells
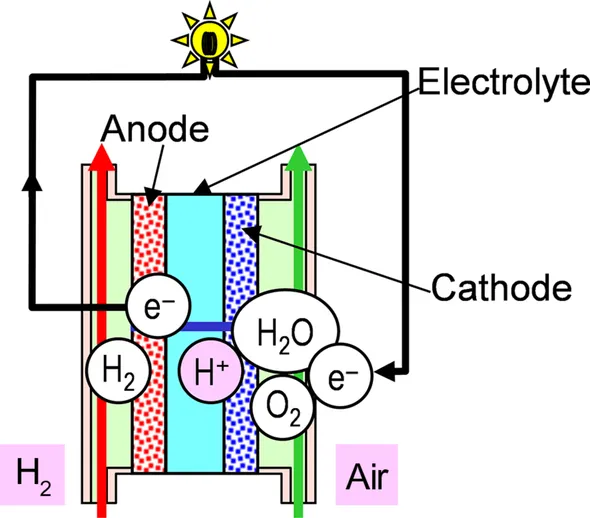
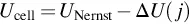
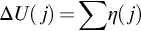
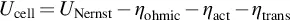
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Contributors
- Preface
- Nomenclature
- Chapter 1: Introduction
- Chapter 2: Irreversible Losses in Fuel Cells
- Chapter 3: Modeling of Polymer Electrolyte Fuel Cells
- Chapter 4: Polymer Electrolyte Fuel Cells
- Chapter 5: Other Polymer Electrolyte Fuel Cells
- Chapter 6: Preparation of MEA
- Chapter 7: Degradation Mechanisms and Their Lifetime
- Chapter 8: Characterization Methods for Components and Materials
- Chapter 9: Electrochemical Measurement Methods and Characterization on the Cell Level
- Chapter 10: Hydrogen Production
- Chapter 11: Role of Hydrogen Energy Carriers
- Chapter 12: Environmental Impact Factors Associated with Hydrogen Energy
- Index