- 500 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
PVC Degradation and Stabilization
About this book
PVC stabilization, the most important aspect of formulation and performance of this polymer, is discussed in details. This book contains all information required to design successful stabilization formula for any product made out of PVC.
Separate chapters review information on chemical structure, PVC manufacturing technology, morphology, degradation by thermal energy, UV, gamma, other forms of radiation, mechanodegradation, and chemical degradation. The chapter on analytical methods used in studying of degradative and stabilization processes helps in establishing system of checking results of stabilization with different stabilizing systems. Stabilization and stabilizers are discussed in full detail in the most important chapter of this book. The final chapter contains information on the effects of PVC and its additives on health, safety and environment.
This book contains analysis of all essential papers and patents published until recently on the above subject. It either locates the answers to relevant questions and offers solutions or gives references in which such answers can be found.
PVC Degradation and Stabilization is must to have for chemists, engineers, scientists, university teachers and students, designers, material scientists, environmental chemists, and lawyers who work with polyvinyl chloride and its additives or have any interest in these products. This book is the one authoritative source on the subject.
- A practical and up-to-date reference guide for engineers and scientists designing with PVC
- Covers thermal, UV, gamma radiation, chemical, and other forms of degradation
- Includes a critical discussion of the sustainability issues faced by PVC and its additives, as well as health and safety concerns
Trusted by 375,005 students
Access to over 1 million titles for a fair monthly price.
Study more efficiently using our study tools.
Information
1
CHEMICAL STRUCTURE OF PVC
From Staudinger’s time it has been known that PVC has a lower thermal stability than comparable low-molecular weight compound such as 1,3,5-trichlorohexane.1 Since then it has been established in many publications2–5 that PVC undergoes chemical change if the temperature exceeds 373K, which is at least 100K less than should be expected based on an analogy to low-molecular weight substances of similar structure such as 1,4,7-trichloroheptane, 2,4-dichloropentadecane, and so on. It was, therefore, quite natural that, from the beginning of studies on PVC thermal stability, irregularities in the polymer chain have been taken into consideration.
Because of the complicated nature of the polymerization reaction mechanisms, numerous structural defects might be expected (see more on this subject in the next chapter). The evaluation of potential dangers of degradation must include polymerization technology, PVC morphological structure, thermal treatment during production and processing, and composition of various additives used in the PVC processing. We will review these subjects in several chapters of this book. This chapter contains information on the chemical structure of polyvinylchloride.
1.1 REPEAT STRUCTURES AND THEIR BASIC ORGANIC CHEMISTRY
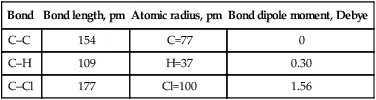
Poly(vinyl chloride) contains three basic bonds: C–C, C–H and C–Cl. Table 1.1 shows the average bonds’ properties.
Table 1.1
Average properties of basic PVC bonds.
| Bond | Bond length, pm | Atomic radius, pm | Bond dipole moment, Debye |
| C–C | 154 | C=77 | 0 |
| C–H | 109 | H=37 | 0.30 |
| C–Cl | 177 | Cl=100 | 1.56 |
The C–C bond length may vary in a broad range; for example, the values of 134 pm and 120 pm have been given for the double bond in alkenes and the triple bond in acetylene compounds, respectively. Also, the presence of double bonds in the close neighborhood of a single bond between two carbon atoms will affect its length (e.g., a single bond in 1,3-butadiene has a length of 148 pm). Single bond length varies depending on its position in α-alkane chain from 152.34 to 152.46 pm (picometers=10−12 m).1a For all-trans n-alkanes, the proton affinities (ΔH298) vary from 142 kcal mol−1 (for ethane) to over 166 kcal mol−1 and increase monotonically from the end of the alkane chain to the center.1a
Similar bond length variations are noted in the case of the C–Cl bond, which may vary in the range of 164–178 pm. The decrease in bond length has been interpreted in terms of about 10–20% double bond character of the C–Cl bond, caused by conjugation of an unshared pair of electrons of the chlorine atom with the double bond or aromatic nucleus. Also, the s-character of the carbon atom results in a bond length change with the highest value for C(sp3)–Cl and the lowest for C(sp)–Cl. Similar changes affect C–C and C–H bond lengths, but they are more pronounced in the case of the C–Cl bond than the C–C bond and the C–H bond. The electronegativity of atoms bound to the carbon atom is responsible for the further changes in the C–Cl bond length. The differences are not large but the effect can influence ground-state stabilization and therefore modify the rates of reaction and equilibrium constants.
Covalent radii, by their nature, are expected to be as sensitive as bond length to hybridization differences at the carbon atoms, electron-delocalization, electronegativity differences, ionic character, and steric effects. There are substantial differences in the character of the three bonds discussed. While the C–C bond is non-polar and the C–H bond only slightly polar, the C–Cl bond is very close to an ionic bond in character. This influences the reactivity of these bonds and the mechanisms of reactions occurring when they are energetically unstable.
It is interes...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Chapter 1: CHEMICAL STRUCTURE OF PVC
- Chapter 2: PVC MANUFACTURE TECHNOLOGY
- Chapter 3: PVC MORPHOLOGY
- Chapter 4: PRINCIPLES OF THERMAL DEGRADATION
- Chapter 5: PRINCIPLES OF UV DEGRADATION
- Chapter 6: PRINCIPLES OF DEGRADATION BY γ-RADIATION
- Chapter 7: DEGRADATION BY OTHER FORMS OF RADIATION
- Chapter 8: MECHANODEGRADATION
- Chapter 9: CHEMICAL DEGRADATION
- Chapter 10: ANALYTICAL METHODS
- Chapter 11: PRINCIPLES OF STABILIZATION
- Chapter 12: HEALTH & SAFETY AND ENVIRONMENTAL IMPACT
- INDEX
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access PVC Degradation and Stabilization by George Wypych in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Industrial & Technical Chemistry. We have over one million books available in our catalogue for you to explore.