
- 340 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Underground Pipeline Corrosion
About this book
Underground pipelines transporting liquid petroleum products and natural gas are critical components of civil infrastructure, making corrosion prevention an essential part of asset-protection strategy. Underground Pipeline Corrosion provides a basic understanding of the problems associated with corrosion detection and mitigation, and of the state of the art in corrosion prevention.
The topics covered in part one include: basic principles for corrosion in underground pipelines, AC-induced corrosion of underground pipelines, significance of corrosion in onshore oil and gas pipelines, numerical simulations for cathodic protection of pipelines, and use of corrosion inhibitors in managing corrosion in underground pipelines. The methods described in part two for detecting corrosion in underground pipelines include: magnetic flux leakage, close interval potential surveys (CIS/CIPS), Pearson surveys, in-line inspection, and use of both electrochemical and optical probes. While the emphasis is on pipelines transporting fossil fuels, the concepts apply as well to metallic pipes for delivery of water and other liquids.
Underground Pipeline Corrosion is a comprehensive resource for corrosion, materials, chemical, petroleum, and civil engineers constructing or managing both onshore and offshore pipeline assets; professionals in steel and coating companies; and academic researchers and professors with an interest in corrosion and pipeline engineering.
- Reviews the causes and considers the detection and prevention of corrosion to underground pipes
- Addresses a lack of current, readily available information on the subject
- Case studies demonstrate how corrosion is managed in the underground pipeline industry
Information
Part I
Understanding and managing corrosion processes
Outline
1 Understanding corrosion in underground pipelines: basic principles
2 AC-induced corrosion of underground pipelines
3 Assessing the significance of corrosion in onshore oil and gas pipelines
4 Numerical simulations for cathodic protection of pipelines
5 Corrosion processes and the use of corrosion inhibitors in managing corrosion in underground pipelines
6 Types of corrosion inhibitor for managing corrosion in underground pipelines
1
Understanding corrosion in underground pipelines: basic principles
R. Norsworthy, Polyguard Products Inc., USA
Abstract:
Corrosion is the deterioration of material or its properties because of a reaction with its environment. Corrosion of pipelines (external, internal, or atmospheric) will depend on the environment and the changes that occur during the life of that pipeline. Basic corrosion principles for metals will be discussed, along with a brief discussion of the typical corrosion mitigation methods. Some of the corrosion mitigation methods are coatings, cathodic protection, and inhibitors.
Key words
anode; cathode; electrolyte; coatings and cathodic protection
1.1 Introduction
The definition of corrosion according to NACE International is ‘The deterioration of a substance or its properties because of a reaction with its environment.’1 All materials can, and do, corrode if we place them in an environment that causes that particular material to deteriorate. Since this book is about metal (usually steel) underground pipelines, the discussion will be about metals. These pipelines are exposed to many different corrosive environments, both internally and externally.
Iron-based metals are the most widely used of all the metals for pipelines. The iron is alloyed with various other metals to produce a variety of steel products that can be formed into pipe of all diameters, wall thicknesses, and lengths. This pipe is connected (usually through welding) and placed in the ground to transport various products a few meters or cross-country to locations of use. These products may be water, oil, natural gas, refined products, or a variety of other liquids, gases, and slurries. The length of the pipeline may be from a few meters to over a thousand kilometers. The diameter of the pipe will vary according to the product type and volume, but can be from a few centimeters to over a meter. Large water pipes can be several meters in diameter. The wall thickness will also vary according to the type of products and pressure requirements. In discussing corrosion processes affecting different types of pipe, this chapter uses the following basic terms and definitions.
1.1.1 Matter
Matter is anything that occupies space. Matter can be a solid, liquid, or gas formed from elements, molecules, chemical compounds, or mixtures.
1.1.2 Elements
An element is a substance that cannot be broken down through chemical reactions; elements are the building blocks of all matter.
1.1.3 Compound
A compound is a combination of two or more elements and is a pure substance having a fixed composition.
1.1.4 Mixture
A combination of elements, compounds, or both held, together by physical rather than chemical forces is a mixture. Unlike a compound, a mixture does not have a fixed composition.
1.1.5 Molecule
A molecule is the smallest particle of an element of compound that retains all the chemical properties of that compound or element.
1.1.6 Atoms
An atom is the smallest chemical unit of an element. An atom consists of a nucleus, which contains positively charged protons, and neutrons, which are neutral in charge. Orbiting around the nucleus of an atom are electrons, which are negatively charged. Each atom is distinguished by the number of protons and electrons. The number of electrons must be equal to the number of protons for an atom to exist.
Figure 1.1 shows the two-dimensional version of a nitrogen atom. Notice the electrons are at different energy levels or orbits around the nucleus. Hydrogen is the smallest of all the atoms and is composed of only one proton and one electron. Helium is the next atom and has two of each. The outer orbit plays a significant role in the corrosion of metals. The simple explanation is that all atoms want to have a full outer shell of electrons. Hydrogen, for example, is on the metal side of the periodic chart in the first column and is a very active atom because it easily gives up electrons to ionize and then seeks electrons to fill the void (acids). Helium on the other hand is called a noble gas and is on the far right hand column of the periodic chart. The noble gases are very stable elements and do not give up or gain electrons because their outer shells are full and they are happy right where they are. The other atoms do not have full outer shell electrons and are therefore seeking to gain or lose electrons to allow them to have a full outer shell. Most elements are found in nature in the ionized state.
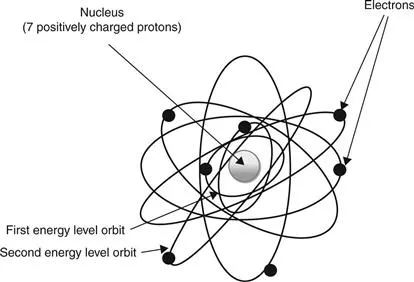
1.1 Bohr model of an atom. Notice that there are two electrons in the first energy level and five in the second energy level.
1.1.7 Ions
Ions are atoms or molecules that have gained or lost electrons. A gain of electrons will create a negatively charged ion called an anion since there are more electrons (negative charges) than protons (positive charges). If there is a loss of electrons the ion is called a cation since there are fewer electrons than protons. Molecules can also become ionized with the gain or loss of electrons. This gain or loss of electrons normally occurs in the outer shell areas of the atom or molecule, but the placement of the electrons take place according to the conditions and elements involved in the process.
1.2 Electrochemical corrosion: conventional current theory
The type of corrosion that occurs on metal pipelines is called ‘electrochemical’. This word provides us with a clue as to what happens in the corrosion process. There is an electrical component (transfer of electrons) and chemical component (oxidation and reduction reactions) that must be present at the same time with equivalent reactions. Each of these topics will be discussed separately. Figure 1.2 shows the basic corrosion cell used to describe the process. Four components must exist at the same time for electrochemical corrosion to occur. These are:
Anode: That part of the structure where conventional current (CC) leaves the metal and enters the electrolyte. Corrosion occurs at the sites where the CC leaves the metal.
Cathode: That part of the structure where CC re-enters the metal from the electrolyte. Protection occurs at the...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Contributor contact details
- Woodhead Publishing Series in Metals and Surface Engineering
- Introduction
- Versatile
- Part I: Understanding and managing corrosion processes
- Part II: Methods for detecting corrosion
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Underground Pipeline Corrosion by Mark Orazem in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Civil Engineering. We have over one million books available in our catalogue for you to explore.