
eBook - ePub
Enzyme Nanoparticles
Preparation, Characterisation, Properties and Applications
- 82 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
This book is the first book in English on nanotechnology and nanomaterials integrating with enzymatic systems, with a focus on nanoparticles and biological applications. It covers comprehensively the relevant topics to understand the development of enzyme nanoparticles as it relates to the complicated structures of enzyme nanoparticles and their functionalization and immobilization on to various supports. The preparation of enzyme nanoparticles, their kinetic properties and applications after immobilization of the immobilized enzyme nanoparticles is described. The use of colour images in all formats of the book will improve the understanding of the topics covered. The book offers an integration of Enzymology and Nanotechnology and provides the latest information on preparation of enzyme nanoparticles, their characterization, their functionalization and immobilization on to various supports and thereafter their kinetic properties and applications in various industries with special reference to Biosensor Technology.
- Focus on enzyme nanotechnology, given the wide appeal of enzymes for diagnostics, therapy and biocatalysis
- Provision of a general background to the topic, but also a detailed description of synthesis, preparation and applications
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Enzyme Nanoparticles by Chandra S. Pundir in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Biochemistry. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
Introduction to Enzyme and Nanotechnology
Materials when reduced down to 1–100 nm (1–100×10−9 m) in their dimension show drastic changes in respect of their physical, chemical, optical, magnetic, mechanical and electrical properties. All these properties lead to exciting applications of these nanomaterials in bioscience, medicine and environmental science, cosmetics, electronics, etc. is called nanotechnology. In other words, nanotechnology is defined as the branch of science and engineering which deals with the design, synthesis, characterization and applications of materials in devices and systems through control of matter on the nanometre scale (normally in the range of 1–100 nm). Different nanostructures have been investigated to determine their properties and possible applications. These structures include nanotubes, nanofibres, nanorods, nanoparticles (NPs) and thin films. Of these nanomaterials, NPs are the best studied. NPs are of two types: organic and inorganic. Among the organic NPs, proteins/enzymes NPs have attracted the attention of researchers during the past decade, specifically their application in the construction of improved biosensors/analytical devices.
Keywords
Cholesterol oxidase; enzyme; enzyme nanoparticles; glucose oxidase; horseradish peroxidase; nanotechnology; nanoparticles; single enzyme nanoparticles; uricase
What Are Nanoparticles and Nanotechnology?
To give an idea of how small things are, the average sizes of some entities are given in Table 1.1 which provides their relative sizes in the following order: Atom > Electron > DNA double helix > Protein/Enzyme > Cell > RBC. Materials when reduced down to 1–100 nm (1–100×10−9 m) in their dimension show drastic changes in respect of their physical, chemical, optical, magnetic, mechanical and electrical properties. All these properties lead to exciting applications of these nanomaterials in bioscience, medicine and environmental science, cosmetics, electronics, etc. that is called nanotechnology. In other words, nanotechnology is defined as the science and engineering that deals with the design, synthesis, characterization and applications of materials in devices and systems through control of matter on the nanometre scale (normally in the range of 1–100 nm). Different nanostructures have been investigated to determine their properties and possible applications. These structures include nanotubes, nanofibres, nanorods, nanoparticles (NPs) and thin films. Of these nanomaterials, NPs are the best studied. NPs are of two types: organic and inorganic. Among the organic NPs, proteins/enzymes NPs have attracted the attention of researchers during the past decade, specifically their application in the construction of improved biosensors/analytical devices.
Table 1.1
Average Size of Some Entities
| Component | Approx. size (nm) |
| Atom | 0.15 |
| Electron | 1 |
| DNA (double helix) | 2 |
| Protein molecule | 10 |
| Cell | 1000 |
| RBC (width) | 7000 |
What Are Enzymes and Their Aggregates?
Enzymes, also known as biological catalyst, are specialized globular proteins (with the exception of a few RNAs called ribozyme), which accelerate the rate of biochemical reaction up to 108 fold by lowering the activation energy (Ea) of the reaction without themselves being consumed in the reaction. Ea is the minimum amount of energy required to bring the substrate(s) from its ground state to transition state. The enzymes are required in small quantities for their biological functions and they possess characteristic kinetic parameters such as optimum pH (the most favourable pH at which the enzyme is most active), incubation temperature (the temperature at which the enzyme activity is maximum), time of incubation (time required to get the maximum activity of an enzyme), Michaelis–Menten constant, Km (the substrate concentration at half of its maximal velocity (Vmax). The portion of enzyme where the substrate binds is called active site. Simple enzymes show hyperbolic relationship between their activity and substrate concentration and follow Michaelis–Menten kinetics during the reaction (v=Vmax[S]/ Km+[S]; where v=initial velocity of reaction). The activity of enzymes can be increased or decreased by certain compounds known as activators and inhibitors, respectively. The inhibition of enzyme is of two types, irreversible and reversible. The reversible inhibition is classified into three types: (i) competitive inhibition, where both substrate and inhibitor (substrate analogue) compete for the same site, i.e. active site of the enzyme, (ii) non-competitive inhibition, where the inhibitor binds with the enzyme (E) or enzyme–substrate (E–S) complex or both at a site other than active site and (iii) uncompetitive inhibition, where the inhibitor binds only with the E–S complex. Enzymes are generally very specific, i.e. they act on a particular substrate. However, some enzymes have absolute specificity, while others have group-specificity, stereo-specificity and geometric specificity. Many enzymes require non-protein substances for their optimum activity, called co-factors. These co-factors are either inorganic or organic or both. The organic co-factors are named as co-enzymes, which are generally the derivatives of vitamins. The enzymes bind with their substrate (S) to form E–S complex, which is finally converted into product (P), due to favourable proximity and favourable orientation, acid–base catalysis, covalent catalysis, electrostatic catalysis, metal ion catalysis (in case of metalloenzymes), strain and distortion and the free enzyme is released. More than 3000 enzymes are known so far. Enzymes are classified into six major groups: EC 1. Oxidoreductases, which catalyse oxidation/reduction reactions, EC 2. Transferases, which catalyse the transfer of functional groups, EC 3. Hydrolases, which catalyse the hydrolysis of various bonds, EC 4. Lyases, which cleave various bonds, non-hydrolytically and nonoxidatively, EC 5. Isomerases, which catalyse isomerization within a single molecule, EC 6. Ligases, which join two molecules covalently at the expense of ATP/GTP. Unit activity (U) of an enzyme is defined as the amount of enzyme/protein required to convert one micro molecule of the S into P per min/ml. The specific activity is the ratio between activity and protein in milligrams. The activity of an enzyme is controlled by many factors such as restricted proteolysis, covalent catalysis (phosphorylation and de-phosphorylation, adenylation and de-adenylation, and acetylation and deacetylation), their end products, adenylate energy charge (ratio of ADP+AMP and ATP), induction and repression. The diameter of a protein/enzyme molecule is approximately 10 nm in solution. However, when the enzyme molecules are aggregated up to 100 nm, they exhibit unique properties such as high surface area, good stability, biocompatibility, but more importantly, high conductivity and sensitivity. These aggregates of enzyme molecules are called enzyme nanoparticles (ENPs).
What Are Enzyme Nanoparticles?
ENPs can be defined as the clustered assembly of enzyme molecules in a fixed form of protein structure in nanometre dimension ranging between 10–100 nm. ENPs show their unique optical, electrical, electronic, thermal, chemical and mechanical, catalytic (ability to facilitate electron transfer) properties besides increased surface area. The ability to tailor the properties of nanoparticles/nanomaterials offers excellent properties for enhancing the performance of enzyme-based sensors. Direct attachment of proteins/enzyme onto nanoparticles may cause their denaturation, thus loss of its activity. To overcome this problem, enzyme molecules have been aggregated to form their nanoparticles and cross-linked within their self in controlled manner, before their immobilization. This has resulted in promising strategy for development of biosensors with improved analytic performance not just in terms of detection limit but also a much larger current response (Sharma et al., 2011). Liu et al. (2005) prepared and characterized NPs of horseradish peroxidase (HRP) for the first time. Thereafter, formation and application of ENPs of glucose oxidase (GOD), cholesterol oxidase (ChOx) and uricase in construction of amperometric biosensors were reported (Sharma et al., 2011; Kundu et al., 2012; Chawla et al., 2013; Chauhan et al., 2014).
The ENPs are different from single enzyme nanoparticles (SENs), which are single enzyme molecules surrounded/armoured by/with a nanoscale organic/inorganic macromolecules network with enhanced catalytic activity and thermal stability. Kim and Grate (2003) prepared SENs of trypsin and chymotrypsin. The resulting SEN deeply preserved the enzyme activity, while enhancing its thermal stability. SENs of HRP and cellulase were also reported recently to get the improved enzyme tolerance at elevated temperature and enhanced cellulose degradation, respectively (Khosravi et al., 2012; Blanchette et al., 2012).
How Are Nanoparticles of Enzymes Prepared?
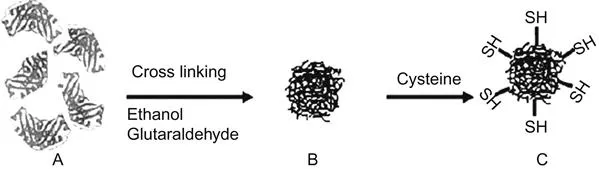
The nanoparticles of the few enzymes such as HRP, GOD, ChOx and uricase have been prepared by desolvation and glutaraldehyde cross-linking method (Liu et al., 2005; Sharma et al., 2011; Kundu et al., 2012; Chawla et al., 2013; Chauhan et al., 2014) (Figure 1.1).
What Does This Book D...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Dedication
- About the author
- Preface
- Chapter 1. Introduction to Enzyme and Nanotechnology
- Chapter 2. Preparation of Enzyme Nanoparticles
- Chapter 3. Characterization of Enzyme Nanoparticles
- Chapter 4. Immobilization of Enzyme Nanoparticles
- Chapter 5. Kinetic Properties of Enzyme Nanoparticles
- Chapter 6. Applications of Enzyme Nanoparticles
- Chapter 7. Future Development in Enzyme Nanoparticles
- Appendix. Enzymes Used in Day-to-Day Life