
- 390 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Dictionary of Toxicology
About this book
Dictionary of Toxicology, Third Edition presents a compendium of definitions of all current toxicological terminology. This authoritative reference illustrates and describes words, concepts, acronyms and symbols for both the toxicological theory and applied risk assessment, as well as providing guidance on the correct selection of problematic, similar and frequently-misused terms.
Written by one of the world's foremost experts in toxicology, and with each entry peer reviewed, Dictionary of Toxicology, Third Edition is an essential reference for all scientific, medical and legal professionals who work with or encounter the toxicological effects of contaminants on biological systems.
New to this edition: an update on every entry and the inclusion of all terminology and concepts relating to molecular toxicology, nanotoxicology and computational toxicology.
- Presents peer-reviewed definitions on the most up-to-date toxicological terms and concepts.
- New edition includes definitions within the fields of molecular toxicology, nanotoxicology, computational toxicology and risk assessment.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Dictionary of Toxicology by Ernest Hodgson,Michael Roe in PDF and/or ePUB format, as well as other popular books in Medicine & Public Health, Administration & Care. We have over one million books available in our catalogue for you to explore.
Information
A
AAALAC See: Association for Assessment and Accreditation of Laboratory Animal Care International.
AAAS See: American Association for the Advancement of Science.
AACT See: American Academy of Clinical Toxicology.
AAFS See: American Academy of Forensic Sciences.
AAG See: α1-acid glycoprotein.
AAPCC See: American Association of Poison Control Centers.
AAS See: spectrometry, atomic absorption.
Abakabi disease See: tricothecenes.
ABCW extrapolation (Obsolete) A method formerly used for extrapolating germ cell mutation tests from species to species. The name is derived from the authors of the initial paper (Abrahamson, Bender, Conger & Wolf, Nature 245, 461, 1973). Based on the results of published studies on the effects of ionizing radiation, the extrapolation was performed by normalizing the mutation rate to the amount of DNA in the haploid genome of the species in question. Attempts to extend the ABCW hypothesis to chemical mutagens were less successful, and the original hypothesis has been challenged even with regard to ionizing radiation. This extrapolation is of historical importance but has been superseded by other techniques. See also: extrapolation; extrapolation, to man; species extrapolation.
ABFT See: American Board of Forensic Toxicology.
abiotic Nonliving; in toxicology used primarily for the nonliving parts of ecosystems.
ABMT See: American Board of Medical Toxicology.
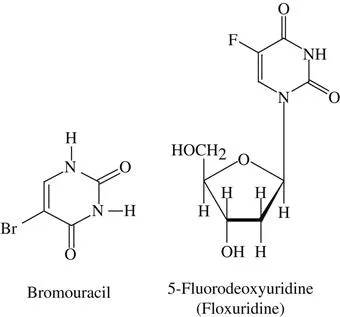
abnormal base analogs Exogenous (xenobiotic) analogs of the bases normally found in DNA, similar enough to substitute for the normal base and give rise to abnormal base pairing. As a result they are potent mutagens. 5-Bromouracil, 5-fluorouridine, 2-aminopurine, and 6-mercaptopurine are examples of mutagenic abnormal base analogs. Incorporation into DNA results in mispairing during the next replication cycle, giving rise to altered (mutant) DNA. Since these chemicals are mutagens and carcinogens, the toxic hazard attendant upon their use is high. See also: carcinogenesis; DNA; 6-mercaptopurine; mutation; therapeutic index. See Figure A1.
abnormal development, consequences Death, malformation, growth retardation, or functional disorders can occur as consequences of abnormal development. The embryo is not usually damaged by most agents prior to differentiation; however, a sufficiently high dose may result in death of the embryo. The time of organogenesis is the most sensitive time for induction of specific malformations, whereas structural defects at the tissue level, growth retardation, or functional deficits are most likely to occur from damage during the fetal period. Structural defects are the main criteria used in estimating teratological risks since they are more obvious. However, functional disorders may be as incapacitating and result in as great a mortality rate among offspring as morphological abnormalities. See also: teratogenesis, critical periods.
ABP See: androgen-binding protein.
ABPI See: Association of the British Pharmaceutical Industry.
abrin (toxalbumin) A lectin composed of two polypeptide chains connected by a disulfide bridge. It is nearly identical to the toxin produced by the castor bean (Ricinus communis). Abrin is found in the seed of the rosary pea (Abrus precatorius), a common vine of the tropics. The LD50 in mice is 0.02 mg/kg, i.p. Ingestion of one chewed or broken seed can be fatal. It is a gastrointestinal toxin; one of the polypeptide chains binds to the intestinal cell membrane, allowing the other chain to enter the cytoplasm. Ribosomal protein synthesis is inhibited, resulting in cell death. Diarrhea associated with bloody mucus may begin as late as 3 days following ingestion of seeds with broken seed coats. Death may occur from loss of intestinal function and consequent alterations in plasma composition leading to secondary cerebral edema and cardiac arrhythmia. Therapy for acute poisoning is to correct hypovolemia and electrolyte balance. See also: castor beans; lectins; ricin.
absinthe A distilled alcoholic drink manufactured from wormwood (Artemisia absinthum), absinthe was widely popular in the late nineteenth and early twentieth centuries especially in France. Although containing minor amounts of neurotoxic materials from the wormwood, it was no more hazardous to human health than other distilled alcoholic drinks. Due to its (probably mistaken) reputation as an addictive drug, by 1915 it was widely banned throughout the western world, including the United States. By the late twentieth century it was no longer prohibited and was once again produced and sold, today enjoying some popularity.
ABVT See: American Board of Veterinary Toxicology.
acaricides Pesticides with specificity for mites, the term being used primarily for phytophagous mites in contrast to parasitic mites, although drugs used for treatment of the latter could be referred to as acaricides. A number of insecticides display acaricidal as well as insecticidal activity. The acaricides include a diverse array of chemical structures. Common examples are dicofol and chlorobenzilate. See also: dicofol.
Acarin See: dicofol.
acceptable daily intake (ADI) The estimated amount of a chemical (usually restricted to pesticides and food additives) that can be ingested daily, by humans, for an entire lifetime without causing appreciable adverse effects; it is expressed in mg/kg body weight/day. The ADI is obtained by dividing the no observed adverse effect level (NOAEL) by an uncertainty factor (e.g. , 10, 100, or 1000) that is intended to make allowance for possible differences in sensitivity between the animal test species and humans, as well as for interindividual variations within the human population. The term ADI was first used by the Joint FAO/WHO Expert Committee on Food Additives in 1961 and subsequently was adopted by the Joint FAO/WHO Expert Committee on Pesticide Residues (1962). The ADI constitutes a useful regulatory benchmark that is employed by international (e.g., FAO/WHO) and national (e.g., EPA, UK Advisory Committee on Pesticides and Other Toxic Chemicals) agencies for establishing tolerances for pesticide residues in raw agricultural and other commodities and for developing health advisory guidelines for such residues in potable water. For regulatory purposes, however, it is being replaced by the USEPA with the reference dose (RfD), also derived from the NOAEL adjusted by uncertainty factors and modifying factors. See also: modifying factor; no observed effect level; reference dose (RfD); uncertainty factor.
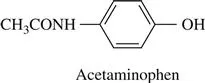
acetaminophen (N-(4-hydroxyphenyl)acetamide; paracetamol; tylenol). CAS number 103-90-2 An analgesic and antipyretic drug. It is also been used in the manufacture of azo dyes and photographic chemicals. The oral LD50 in mice is 338 mg/kg and the i.p. LD50 is 500 mg/kg. Acetaminophen is a hepatotoxicant at high doses and, in some developed countries is the leading cause of acute liver failure, primarily as a result of deliberate (suicidal) overdose. Toxicity is due to the metabolism of acetaminophen to a toxic intermediate, N-acetyl-p-benzoquinonimine (NAPQI) by cytochrome P450. Following an overdose, detoxication of NAPQI by glutathione conjugation is saturated, leading to an increase in the concentration of the toxic metabolite that, in turn, binds to various hepatocellular constituents. Within hours sweating, anorexia, nausea, and vomiting develop. In 3–5 days, jaundice, coagulation defects, hypoglycemia, renal failure, and myocardiopathy may occur. If given within 12 h of ingestion of an acetaminophen overdose, N-acetylcysteine appears to be effective in blocking the covalent binding of the toxic metabolite and the prevention of hepatotoxicity. See Figure A2.
Reference: Hinson, J. A. et al. Drug Metabol Rev. 36, 805–822, 2004.
Reference: Hinson, J. A. et al. Drug Metabol Rev. 36, 805–822, 2004.
acetazolamide ((N-(5-)aminosulfonyl)-1,3,4-thiadiazole-1,2-yl) acetamide; 5-acetamido-1,3,4-thiadiazole-2-sulfonamide; Diamox). CAS number 59-66-5 Acetazolamide is...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- About the Editors
- Contributors to the Third Edition
- Contributors to the First and Second Editions
- Preface
- Introduction
- Abstract and Keywords
- A
- B
- C
- D
- E
- F
- G
- H
- I
- J
- K
- L
- M
- N
- O
- P
- Q
- R
- S
- T
- U
- V
- W
- X
- Y
- Z