
- 308 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Soap Manufacturing Technology
About this book
Soap Manufacturing Technology, Second Edition, is the most authoritative and up-to-date book on soap technology available today. Editor and contributing author Luis Spitz leads a world-renowned team in providing comprehensive information on all components of soap manufacturing including formulation, performance evaluation, cleansing systems, and more. This new edition includes two new chapters, Integrated Saponification and Drying Systems and Laundry Bars, and the others are completely revised and updated.
- Includes new chapters and figures, tables, and text updated from the first edition
- Serves as a technical reference book ideal for both experienced and beginning soap producers and suppliers
- Provides an overview of the AOCS methods used for the evaluation of soap and soap products
- Includes two new chapters on Integrated Saponification and Drying Systems and Laundry Bars
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Soap Manufacturing Technology by Luis Spitz in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Chemical & Biochemical Engineering. We have over one million books available in our catalogue for you to explore.
Information
Edition
21
Implications of Soap Structure for Formulation and User Properties
Norman Hall Continua Consulting Service, United Kingdom
Basic Chemistry
The fundamental principles of soap composition and performance are generic and apply to all soaps. The chemistry of soapmaking is very simple. Soaps are the salts of (mainly) saturated and unsaturated fatty acids having carbon number C10 to C18. The source of the fatty acids is always a blend of natural triglyceride oils. However, relatively few manufacturers make soap by neutralizing a blend of fatty acids. Most create soap directly from the blend of oils.
To make soap directly from oil, a blend of glyceride oils is reacted with a strong sodium hydroxide solution to give the soap, plus glycerine—and a lot of heat. Separating the soap from the glycerine byproduct is not easy, and may not even be necessary.

The alternative is to split the triglyceride oil into fatty acids and glycerine using high temperatures and high pressures. In this case the fatty acids and glycerine can easily be separated. The separated fatty acids are normally distilled, blended, and then neutralized with NaOH solution to form soap.

Whether it is better to make soap by classical saponification or by a fatty acids route involves economics, supply chain issues, raw material, and finished product qualities. The availability of equipment and of operators skilled in the relevant processes can be important issues to consider.
Glycerides
The alkyl chains R1, R2, and R3 on the triglyceride molecule of the oil or fat include both saturated and unsaturated fatty acid types, of different carbon atom chain lengths (carbon numbers), for example:

This example shows a triglyceride with two saturated fatty acid chains (say, palmitic acid, C16) and one unsaturated fatty acid chain (say, oleic acid, C18:1). Several different combinations of saturation/unsaturation and chain length are possible on a given glycerine backbone, resulting in oils that differ widely in their characteristics—especially their melting points. These differences are very important for edible applications, where the oils are used as triglycerides, but are much less important for soap applications, because in soapmaking all the fatty acid chains are separated from the glycerine backbone.
For soaps the only important factors are the relative proportions of saturated to unsaturated fatty acids (measured by the iodine value—the grams of iodine reacting with the unsaturated component in 100 g of oil or fat), and the lengths of the fatty acid chains (carbon number).
The proportions of saturated and unsaturated fatty acids and the chain lengths of those acids are characteristics of the oil or oil blend used for making soap.
Why Use a Mixture of Oils?
A blend of oils is almost always used to make toilet soaps or laundry soaps. The most common oils are coconut oil (CNO) or palm kernel oil (PKO), which, understandably, are generally called nut oils, and tallows (AT or T) or palm oils (PO), which are generally called non-nut oils. To get the best performance from soaps, you need both nut oils and non-nut oils.
The Non-Nut Oils (Tallows or Palm Oils)
These oils provide long-chain-length saturated fatty acids (C16/C18—palmitic and stearic acids), resulting in soaps that are almost insoluble at normal user temperatures and therefore do not lather. To put this insolubility into context, consider that calcite is more soluble in water at 25 °C than is sodium stearate. However, these almost insoluble soaps add to lather stability and add hardness, which makes the soap solid.
Lather stability is to a large extent governed by the rate at which a liquid film drains under gravity from between the bubbles. When the liquid film becomes so thin at any point that the film sides touch, then the bubbles burst. Any mechanism that slows the rate of liquid drainage will increase lather stability.
When the liquid film contains insoluble particles, these can sometimes “bridge” the film sides and cause premature lather instability. This can happen with very large particles of filler materials. However, the particles of insoluble soaps are very small, and they are asymmetric. There are two consequences of this asymmetry.
First, in the same way that sticks of wood flowing in a fast-moving river will align with their longest axis parallel to the river flow, so the insoluble, asymmetric particles of sodium stearate and sodium palmitate will align so that their long axis is parallel to the flow of liquid draining from between the bubbles of lather. The narrow axis of the stearate/palmitate particles is very small, so it is unlikely that the particles will bridge the film sides until most of the liquid has drained.
Second, and most important, at the junctions between the air bubbles there is a region of much slower liquid flow. This is the Gibbs-Plateau border. In this region, motion of the particles of sodium stearate/palmitate become much more random, like wooden sticks floating randomly in a slow-moving pond of water.
Just as sticks floating in a slow pond can cause a log jam that inhibits the water flow, so the particles of sodium stearate/palmitate can collect in the the Gibbs-Plateau border to an extent that they will block the flow of liquid draining from the lather. This increases the time it takes for the bubble film sides to touch and for the bubbles to burst—and so increases lather stability
Tallow or palm oils also provide long-chain-length unsaturated fatty acids (mainly C18:1), and the chain length gives soaps with reasonable solubility but only moderate lather stability and rather poor lather volume. However, the moderate lather stability of sodium oleate from tallow or palm oils is improved significantly by the lather-stabilizing effect of the insoluble sodium stearate/palmitate soaps provided by the same oils. This means tallow soap or palm oil soap alone can give a reasonable amount of lather and have good lather stability, provided the use temperature is high enough (over 25–30 °C) to dissolve the sodium oleate.
The Nut Oils
These oils provide short-chain-length fatty acids (especially C12, lauric acid) that result in soaps of moderate solubility, but with high lather when they are dissolved. Solubility can be increased by using the soap at a higher temperature, but sodium laurate does not dissolve significantly until the temperature is over 40 °C.
The Importance of the Oleate:Laurate Eutectic Mixture
It is a common but mistaken idea that nut oils make soap lather because the C12 soap has a high lather. It is true that C12 soap has a higher lather than C18 soap (which effectively does not lather at all) and a higher lather than C18:1 soap. However, the lather of a soap containing both tallow/palm and nut oil is much higher than that of C12 soap alone. The reason for this is fundamental to many aspects of the performance of soap tablets.
When a system contains both C12 and C18:1 fatty acid soaps together, the mixture has a solubility that is much higher than the solubilities of either of the individual components. For example, a 1:1 mixture of C12 sodium soap and C18:1 sodium soap is very soluble and has a very high lather. This mixture will also make the soap softer. Consider the Krafft temperatures (Tk) of
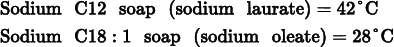
The Krafft temperature is the temperature, or more precisely the narrow temperature range, above which the solubility of a surfactant increases rapidly. At this temperature the solubility becomes equal to the critical micelle concentration. When micelles form in the solution, the detergent can diss...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Preface
- 1: Implications of Soap Structure for Formulation and User Properties
- 2: Soap Structure and Phase Behavior
- 3: Formulation of Traditional Soap Cleansing Systems
- 4: Chemistry, Formulation, and Performance of Syndet and Combo Bars
- 5: Transparent and Translucent Soaps
- 6: Semi-Boiled and Integrated Saponification and Drying Systems
- 7: Soap Drying Systems
- 8: Bar Soap Finishing
- 9: Soap, Soap/Synthetic, and Synthetic Laundry Bars
- 10: Multicolored and Multicomponent Soap Manufacturing Systems
- 11: Soap Bar Performance Evaluation Methods
- 12: Glossary
- Index