
Application of Nuclear Magnetic Resonance Spectroscopy in Organic Chemistry
International Series in Organic Chemistry
- 472 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Application of Nuclear Magnetic Resonance Spectroscopy in Organic Chemistry
International Series in Organic Chemistry
About this book
Applications of Nuclear Magnetic Resonance Spectroscopy in Organic Chemistry, Second Edition focuses on the applications of nuclear magnetic resonance spectroscopy to problems in organic chemistry and the theories involved in this kind of spectroscopy. The book first discusses the theory of nuclear magnetic resonance, including dynamic and magnetic properties of atomic nuclei, nuclear resonance, and relaxation process. The manuscript also examines the experimental method. Topics include experimental factors that influence resolution and the shapes of absorption lines; measurement of line positions and identification of the chemical shift; and measurement of intensities. The text reviews the theories of chemical effects in nuclear magnetic resonance spectroscopy and spin-spin multiplicity and the theory and applications of multiple irradiation. The book also tackles the theory of chemical shift, including the classification of shielding effects, local diamagnetic proton shielding, solvent effects, and contact shifts. The publication is a dependable source of data for readers interested in the applications of nuclear magnetic resonance spectroscopy.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
THEORY OF NUCLEAR MAGNETIC RESONANCE
Publisher Summary
A DYNAMIC AND MAGNETIC PROPERTIES OF ATOMIC NUCLEI
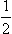
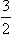
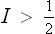
B NUCLEAR RESONANCE
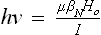
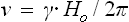
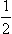
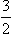
Table of contents
- Cover image
- Title page
- Table of Contents
- INTERNATIONAL SERIES IN ORGANIC CHEMISTRY
- Copyright
- Dedication
- PREFACE
- PREFACE TO THE 1st EDITION
- EDITORIAL PREFACE TO THE 2nd EDITION
- PART 1: An introduction to the theory and practice of nuclear magnetic resonance spectroscopy
- PART 2: Theory of chemical effects in nuclear magnetic resonance spectroscopy
- PART 3: Applications of the chemical shift
- PART 4: Spin-spin coupling
- PART 5: Applications of time-dependent phenomena
- REFERENCES
- INDEX