![]()
1
Overview of Plant Polymers
Resources, Demands, and Sustainability
Outline
1.1 Plant Proteins
1.2 Plant Oils
1.3 Plant Starches
1.4 Agricultural Fibers and Cellulose
1.5 Market Potential for Plant Polymers
1.6 Sustainable Agriculture Industry of the Future
1.7 Conclusion
Acknowledgment
References
Adapted from a chapter in: Wool, Bio-based Polymers and Composites (2005).
Advances in petroleum-based fuels and polymers have benefited mankind in numerous ways. Petroleum-based plastics can be disposable and highly durable, depending on their composition and specific application. However, petroleum resources are finite, and prices are likely to continue to rise in the future. In addition, global climate change, caused in part by carbon dioxide released by the process of fossil fuel combustion, has become an increasingly important problem, and the disposal of items made of petroleum-based plastics, such as fast-food utensils, packaging containers, and trash bags, also creates an environmental problem. Petroleum-based or synthetic solvents and chemicals are also contributing to poor air quality. It is necessary to find new ways to secure sustainable world development. Renewable biomaterials that can be used for both bioenergy and bioproducts are possible alternatives to petroleum-based and synthetic products.
Agriculture offers a broad range of commodities, including forest, plant/crop, farm, and marine animals, that have many uses. Plant-based materials have been used traditionally for food and feed and are increasingly being used in pharmaceuticals and nutraceuticals. Industrial use of agricultural commodities for fuels and consumer products began in the 1920s, but they were soon replaced by petroleum-based chemicals after World War II because of low cost and durability of petrochemicals. This chapter focuses on bio-based polymers derived from plant-based renewable resources, their market potential, and the sustainability of the agriculture industry of the future.
The three major plant-based polymers are protein, oil, and carbohydrates. Starch and cellulose, also called polysaccharides, are the main naturally occurring polymers in the large carbohydrate family. Agricultural fiber is also a member of the carbohydrate family. Natural fiber such as flax, hemp, straw, kenaf, jute, and cellulose consists mainly of cellulose, hemicellulose, and lignin, but is usually listed as a material when used as a fiber in composites.
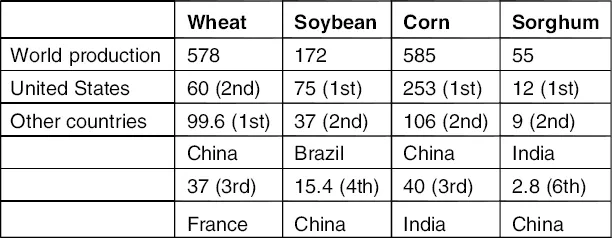
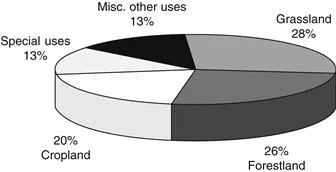
Corn, soybean, wheat, and sorghum are the four major crops grown in the United States (Table 1.1), with total annual production of about 400 million metric tons (800 billion pounds) in the year 2000. Annually, 10–15% of these grains are used for food, 40–50% for feeds, and the rest could be for various industrial uses. Based on U.S. Department of Agriculture statistics, the total land used for crops is about 455 million acres, which is about 20% of the total usable land (Fig. 1.1) [1]. Including other crops, such as rice, barley, peanuts, and canola, the United States has the potential to produce about 550 million metric tons of grains and legumes. At least 150 million metric tons of grains and legumes are available for nonfood industrial uses. In general, seeds make up about 45–52% of the dry mass of a plant. This means that there is the potential to produce about 400 million metric dry tons of cellulosic sugar-based biomass (agriculture fiber residues) annually in the United States alone based on the total production of corn, soybean, wheat, and sorghum. Including other crops, plants, and forest products, the total annual US production of cellulosic sugar-based biomass could be about 800 million dry tons.
Table 1.1 Production of Selected Grains and Legumes (Million Metric Tons)
Sources: From Ref. [31] and USDA World Agriculture Production, July 27, 2001.
Figure 1.1 Land use and distribution. Total useful land in the United States is about 2.3 billion acres.
1.1 Plant Proteins
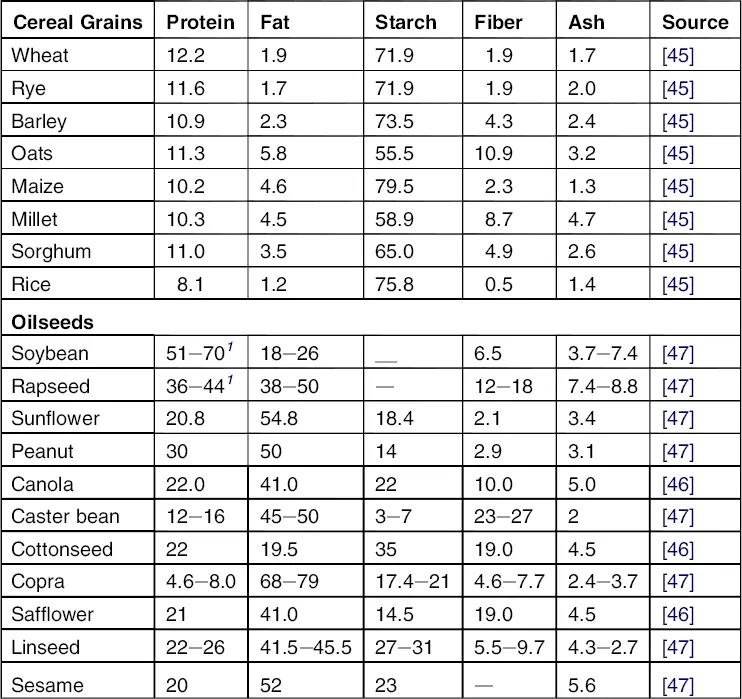
Plant proteins are amino acid polymers derived mainly from oilseeds (i.e., soybeans) and grains (i.e., wheat and corn), and are usually produced as by-products of processing oils and starches (Table 1.2). The potential US protein production is about 120 billion pounds of soybean meal containing about 50% protein, about 20 billion pounds of wheat gluten containing about 70% protein, and about 40 billion pounds of corn gluten containing about 65% protein. Of the corn protein, about 30% is a functional protein called corn zein protein [2]. Plant proteins are widely used as major ingredients for food, feed, pharmaceuticals, nutraceuticals, paper coating, textile sizing, and, increasingly, adhesives. Plant proteins are complex macromolecules that contain a number of chemically linked amino acid monomers, which together form polypeptide chains, constituting the primary structure. The helix and sheet patterns of the polypeptide chains are called secondary structures. A number of side chains are connected to the amino acid monomers. These side chains and attached groups interact with each other, mainly through hydrogen and disulfide bonds, to form tertiary or quaternary structures. These proteins often have large molecular weights, in the range of 100,000–600,000 Dalton (Da) (Dalton = grams per mole), which makes them suitable for polymers and adhesives.
Table 1.2 Average Composition of Cereal Grains and Oilseeds (% Dry Weight Basis)
Sources: From Refs. [45], [46], and [47].
Proteins can be modified by physical, chemical, and enzymatic methods. Modification results in structural or conformational changes from the native structure without alteration of the amino acid sequence. Modifications that change the secondary, tertiary, or quaternary structure of a protein molecule are referred to as denaturation modifications [3]. The compact protein structure becomes unfolded during denaturation, which is accompanied by the breaking and reforming of the intermolecular and intramolecular interactions [4].
Physical modification methods mainly involve heat [5] and pressure [6] treatments. Heat provides the protein with sufficient thermal energy to break hydrophobic interactions and disassociate the subunits [5]. The disassociation and unfolding expose the hydrophobic groups previously enclosed within the contact area between subunits or on the interior of the folded molecules. For example, soybean protein disassociates and coagulates at high pressure and exhibits large hydrophobic regions and high viscosity [6].
Chemical modification methods may cause alteration of the functional properties, which are related closely to protein size, structure conformation, and the level and distribution of ionic charges. Furthermore, chemical treatments could cause reactions between functional groups, resulting in either adding a new functional group or removing a component from the protein. Chemical modification methods include acetylation, succinylation, phosphorylation, limited hydrolysis, and specific amide bond hydrolysis. Acetylation is the reaction between a protein amino, or a hydroxyl group, and the carboxyl group of an acetylating agent. The acetylation reaction can modify the surface hydrophobicity of a protein [7]. Succinylation converts the cationic amino groups in the protein to an anionic residue, which increases the net negative charge, resulting in an increase in hydrophobicity under specific succinylating conditions [8]. This treatment also increases the viscosity [9]. Phosphorylation is another effective method to increase negative charges, thereby affecting gel-forming ability and cross-linking [10]. Gel-forming ability can also be increased by alkylation treatment [8]. Chemical hydrolysis is one of the most popular methods for protein modifications by acid-based agents. For example, peptide bonds on either side of aspartic acid can be cleaved at a higher rate than other peptide bonds during mild acid hydrolysis [11]. The hydrophobicity of a protein greatly increases under specific conditions of mild acid hydrolysis [12, 13].
1.2 Plant Oils
Plant oils, such as soy oil, corn oil, and flax oil, can be derived from many crops (Table 1.2). The United States has the potential to produce about 30 billion pounds of soy oil, 25 billion pounds of corn oil, and many billion pounds of oils from other oilseeds as listed in Table 1.2. Plant oils are triglycerides and contain various fatty acids. Soybean, a major oil plant, contains about 20% oil. Soy oil is inexpensive in the United States, sold at about $0.20/lb. Refined soy oil contains more than 99% triglycerides and about eight major fatty acids, including linoleic, oleic, linolenic, palmitic, and stearic acids [14]. These fatty acids differ in chain length, composition, distribution, and location. Some are saturated, and some are unsaturated, which results in differences in the physical and chemical properties of the oil. Control of the fatty acid distribution function is essential to optimize polymer properties. Such plant oil can be physically treated and chemically modified to meet specific industrial applications [15].
Adhesives and resins can be derived from bio-based oils using similar synthetic techniques to those used with petroleum polymers. Many active sites from the triglycerides, such as double bonds, allylic carbons, and ester groups, can be used to introduce polymerizable groups. Wool and coworkers [16] prepared soy oil-based resins by functionalizing the triglycerides. This was accomplished by attaching polymerizable chemical groups, such as maleinates and acrylic acid, or by converting the unsaturated sites to epoxies or hydroxyl functionalities, making the triglycerides capable of polymerizing via ring-opening, free-radical, or polycondensation reactions.
The second method of producing resins from oil is to reduce the triglycerides into monoglycerides. Polymerizable groups, such as maleate half esters, can be attached to the monoglycerides, allowing them to polymerize through free-radical polymerization [17].
The third method is to functionalize the unsaturated sites and reduce the triglycerides to monoglycerides, which can form monomers by reacting with maleic anhydride, allowing polymerization by free-radical polymerization [18,19]. Such reactions produce bio-based polymers that have properties and costs comparable to those of petrochemical-based adhesives and composite resins.
1.3 Plant Starches
Starch is a carbohydrate polymer t...