
eBook - ePub
Cathodic Corrosion Protection Systems
A Guide for Oil and Gas Industries
- 492 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Corrosion is a naturally occurring cost, worth billions in the oil and gas sector. New regulations, stiffer penalties for non-compliance and aging assets are all leading companies to develop new technology, procedures and bigger budgets catering to one prevailing method of prevention, cathodic protection. Cathodic Corrosion Protection Systems: A Guide for Oil and Gas Industries trains on all the necessary reports, inspection criteria, corrective measures and critical standards needed on various oil and gas equipment, structures, tanks, and pipelines. Demands in the cathodic protection market have driven development for better devices and methods, helping to prolong the equipment and pipeline's life and integrity. Going beyond just looking for leaks, this handbook gives the engineer and manager all the necessary tools needed to put together a safe cathodic protection system, whether it is for buried casing while drilling, offshore structures or submarine pipelines.
- Understand how to install, inspect and engage the right cathodic protection systems for various oil and gas equipment, tanks, and pipelines
- Properly construct the right procedure and anodes with all relevant US and International standards that apply
- Gain knowledge concerning techniques, equipment, measurements and test methods used in real-world field scenarios
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Cathodic Corrosion Protection Systems by Alireza Bahadori in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Industrial Management. We have over one million books available in our catalogue for you to explore.
Information
1
Principle of Electrochemical Corrosion and Cathodic Protection
Abstract
To protect pipelines and other metal structures from corrosion, the oil and gas industry uses cathodic protection (CP). The science of CP is based on electrochemistry. It is complex, but in short, CP suppresses unwanted corrosion reactions by applying a protective electrical current. CP provides an effective method of mitigating the corrosion damage to metal surfaces exposed to a conducting (corrosive) electrolyte. This chapter provides the design requirements for electrochemical corrosion and CP as well as the electrochemical methods of preventing corrosion that consist of cathodic and anodic protection.
Keywords
Cathodic protection; Galvanic; Impressed current systems; Passivity; Polarization; Sacrificial anodeCathodic protection (CP) is a technique used to control the corrosion of a metal surface by making it the cathode of an electrochemical cell. The simplest method to apply CP is by connecting the metal to be protected with a piece of another more easily corroded “sacrificial metal” to act as the anode of the electrochemical cell. The sacrificial metal then corrodes instead of the protected metal. For structures in which passive galvanic CP is not adequate, for example, in long pipelines, an external direct current (DC) electrical power source is sometimes used to provide current.
CP systems are used to protect a wide range of metallic structures in various environments. Common applications are steel water or fuel pipelines and storage tanks, such as home water heaters; steel pier piles; ship and boat hulls; offshore oil platforms; onshore oil well casings; and metal reinforcement bars in concrete buildings and structures. Another common application is in galvanized steel, in which a sacrificial coating of zinc on steel parts protects them from rust.
CP can, in some cases, prevent stress corrosion cracking. If two dissimilar metals are touching and an external conducting path exists, corrosion of one of the metals can take place. Moisture or other materials acting as an electrolyte between the metals create an electrochemical cells (similar to that of a battery). Depending on the metals, one will act as a cathode and one will act as an anode of the cell.
Under this arrangement, stray DC currents will flow. In the same way in a normal cell, an electrochemical reaction takes place, and there is a resulting corrosion of the anode.
CP works by converting all anodes that are likely to corrode the cathodes. There are two principal methods of doing this:
1. by attaching a more active metal to form a new anode (making the existing anode the cathode), resulting in the new material (sacrificial anode) being corroded rather than the protected material;
2. by injecting a DC current (impressed current), which uses an anode connected to an external DC source to provide the protection.
CP provides an effective method of mitigating the corrosion damage to metal surfaces exposed to a conducting (corrosive) electrolyte.
• Types of CP
A galvanic sacrificial anode attached to the hull of a ship is shown in Fig. 1.1.

• Galvanic anode
In the usual application, a galvanic anode, a piece of a more electrochemically “active” metal, is attached to the vulnerable metal surface where it is exposed to the corrosive liquid. Galvanic anodes are designed and selected to have a more “active” voltage (more negative electrochemical potential) than that of the metal of the target structure (typically steel).
For effective CP, the potential of the steel surface is polarized (pushed) more negatively until the surface has a uniform potential. At that stage, the driving force for the corrosion reaction with the protected surface is removed. The galvanic anode continues to corrode; this consumes the anode material until it must eventually be replaced. Polarization of the target structure is caused by the electron flow from the anode to the cathode, so the two metals must have a good electrically conductive contact. The driving force for the CP current is the difference in the electrochemical potential between the anode and the cathode.
Galvanic or sacrificial anodes are made in various shapes and sizes using alloys of zinc, magnesium, and aluminum. American Society for Testing and Materials International publishes standards on the composition and manufacturing of galvanic anodes.
In order for galvanic CP to work, the anode must possess a lower (i.e., more negative) electrochemical potential than that of the cathode (the target structure to be protected).
• Impressed current systems
In the simple impressed current CP (ICCP) system, a source of DC electric current is used to help drive the protective electrochemical reaction.
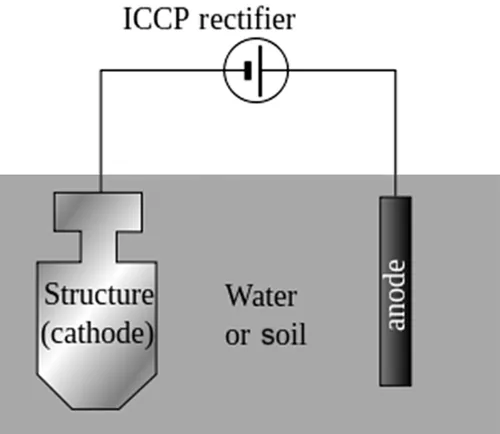
For larger structures, galvanic anodes cannot economically deliver enough current to provide complete protection. ICCP systems use anodes connected to a DC power source, often a rectifier from a local alternating current (AC) system (Fig. 1.2). In the absence of an AC supply, alternative power sources, such as solar panels, wind power, or gas powered thermoelectric generators, may be used. For example, all telephone lines are biased to −36 to −60 V compared to the earth, to reduce galvanic corrosion.
Anodes for ICCP systems are available in a variety of shapes and sizes. Common anodes are tubular and solid rod shaped or are continuous ribbons of various materials. These include high silicon, cast iron, graphite, mixed metal oxide, platinum and niobium-coated wires, and other materials.
For pipelines, anodes are arranged in ground beds either distributed or in deep vertical holes depending on several design and field condition factors, including current distribution requirements.
Rectifier units are often custom manufactured and equipped with a variety of features, including oil cooling, automatic output adjustment, various types of electrical enclosures, remote monitoring, remote output adjustment, an AC electrical outlet, selectable AC input setting, and three-phase AC input. The rectifier output DC negative terminal is connected to the structure to be protected by the CP system. The rectifier output DC-positive cable is connected to the auxiliary anodes. The AC power cables are connected to the rectifier input AC cable terminals.
The output of the rectifier is usually determined by a CP expert to optimize the level of protection on the target structure. Many rectifiers are designed with taps on the transformer windings and jumper terminals to vary the voltage output of the rectifier unit. Rectifiers for water tanks and those used in other applications are made with solid-state circuits to automatically adjust the operating voltage to maintain a target current output or structure-to-electrolyte potential. Analog or digital meters are often installed to show the operating voltage (DC and sometimes AC) and current output.
The principle of ICCP forces the structure to be protected to become the cathode by connection to an anode and injection of a DC. The DC power supplies typically vary the current to achieve the required protection potential (Fig. 1.3).
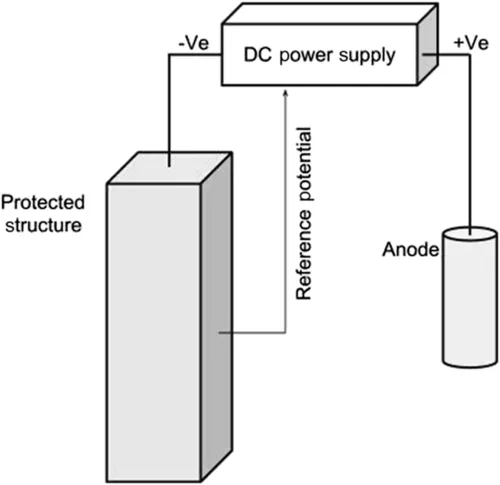
In ICCP systems, anodes can range from low-end consumable metals to more exotic materials that will exhibit little or no corrosion.
• Sacrificial Anode
This is the practice of using a more active metal (sacrificial anode) connected to a structure to be protected, knowing that this metal will be corroded. One example of this would be the use...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Dedication
- Biography
- Preface
- Acknowledgments
- 1. Principle of Electrochemical Corrosion and Cathodic Protection
- 2. Application of Cathodic Protection
- 3. Design Considerations on Cathodic Protection for Buried Pipelines and Marine Structures
- 4. Materials and Cathodic Protection Systems
- 5. Monitoring Cathodic Protection Systems
- 6. Test Methods
- 7. Installation of Cathodic Protection Systems
- 8. Commissioning of Cathodic Protection Systems
- Additional List of Reading on Retrofitting
- Glossary of Terms
- Index