
eBook - ePub
Surface Modification of Magnesium and its Alloys for Biomedical Applications
Modification and Coating Techniques
- 460 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Surface Modification of Magnesium and its Alloys for Biomedical Applications
Modification and Coating Techniques
About this book
The development of biodegradable implants which can remain in the human body to fix a problem and subsequently dissolve, or be absorbed, consumed or excreted, without warranting a secondary surgery, is very appealing to scientists. Due to their excellent biocompatibility and biodegradability, magnesium implants provide a viable option many problems associated with permanent metallic implants such as, restenosis, thrombosis, permanent physical irritation, and inability to adapt to growth and changes in human body. Volume 2 of this important new book explores practical issues of magnesium and magnesium alloys, physical and mechanical modification and coatings to enhance this material for biomedical applications.
- Includes expert analysis on chemical solution deposition of hydroxyapatite (HAp) and octacalcium (OCP) phosphate coatings for magnesium
- Comprehensive coverage of biomimetic modifications, surface functionalization of biomolecules, natural, conducting and biodegradable polymeric coatings
- Lucid dissection of chemical, physical, mechanical and electromechanical modifications of magnesium and its alloys for biomedical applications
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Surface Modification of Magnesium and its Alloys for Biomedical Applications by T.S.N. Sankara Narayanan,Il-Song Park,Min-Ho Lee in PDF and/or ePUB format, as well as other popular books in Medicine & Medical Technology & Supplies. We have over one million books available in our catalogue for you to explore.
Information
Part One
Chemical and physical modifications of magnesium and its alloys for biomedical applications
1
Fluoride conversion coatings for magnesium and its alloys for the biological environment
Thiago F. da Conceição1, and Nico Scharnagl2 1Departamento de Química - CFM, UFSC, Campus Trindade, Florianópolis/SC, Brazil 2Helmholtz-Zentrum Geesthacht Centre for Materials and Coastal Research, Geesthacht, Germany
Abstract
Studies in the literature have shown that magnesium fluoride is an interesting coating for protecting magnesium and magnesium alloys from corrosion in biological environments. In this chapter, this coating formation process and its relevant properties are discussed in detail. The corrosion performance of magnesium alloys with fluoride coating in regular saline solution, simulated body fluid, and in vivo is described. The current challenges and future trends are critically discussed based on recent reports in the literature.
Keywords
Corrosion; Fluoride conversion coating; Magnesium1.1. Introduction
In the last few years, magnesium and its alloys have been considered as biodegradable materials for applications such as orthopedic implants and stents for vessel dilatation (Witte, 2010; Virtanen, 2011). Due to its biocompatibility (of magnesium and of its corrosion products) and good mechanical properties, magnesium-based implants may serve as load-bearing devices, contributing to the healing of the organism and then gradually degrading without causing adverse effects. Among the beneficial effects of such devices is that surgery for implant removal is not required. However, magnesium alloys have low corrosion resistance in aqueous environments containing ions such as Cl−, like the biological environment. Therefore, magnesium implants in such mediums may undergo earlier failure, excessive hydrogen production (which forms gas cavities and inflammation), and a high pH increase in the neighborhood of the implant, causing postoperatory complications (Virtanen, 2011).
The literature reports numerous surface treatments and coatings to control magnesium corrosion in biological environments (Hornberger, Virtanen, & Boccaccini, 2012). Among these, the preparation of fluoride conversion coatings has received special attention. The most studied fluoride conversion coating on magnesium is magnesium fluoride (MgF2), which can be easily prepared by immersion of magnesium in a solution containing fluoride anions, as hydrofluoric acid (HF) solutions. Reports in the literature suggest that, besides increasing corrosion resistance, this conversion coating has antibacterial properties (Lellouche, Friedman, Lelouche, Gedanken, & Banin, 2012; Lellouche, Kahana, Elias, Gedanken, & Banin, 2009) and may induce bone healing due to beneficial effects of fluoride (Berglundh, Abrahamsson, Albouy, & Lindhe, 2007). In this chapter, the formation of MgF2 coating on magnesium alloys is discussed. The potential of this treatment to protect magnesium from corrosion and to enhance its corrosion resistance in biological environments is considered in detail, as well as future trends and current challenges.
1.2. Coating formation: Mechanism and characteristics
1.2.1. Hydrofluoric acid immersion
The traditional method for preparing fluoride coating on magnesium and its alloys is to immerse the sample in an aqueous solution of hydrofluoric acid for a time period that can vary from a few minutes to days. It is generally assumed that the coating is formed by the reaction of magnesium with HF, as shown in Eqn (1.1). This reaction has a negative change in the Gibbs free energy, indicating that this is a product-favored reaction (the change in free energy was obtained using the data in chemical thermodynamic tables, in the temperature of 298.15 K, reported by Wagman et al., 1982). The literature reports different conditions for this treatment in regard to treatment time, acid concentration, and substrate pretreatment. Table 1.1 shows some characteristics of HF treatment described in the literature for different magnesium alloys. A systematic investigation on solution concentration and treatment time on the coating properties is reported in detail by Conceição, Scharnagl, Blawert, Dietzel, and Kainer (2010), Verdier, Laak, Delalande, Metson, and Dalard (2004), and Bakhsheshi-Rad, Idris, Kadir, and Daroonpavar (2013). Coating properties such as thickness, constitution, and porosity change significantly depending on these parameters.
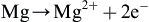
In the studies of Conceição et al. (2010) and Verdier et al. (2004) it was shown that the coating can present a considerable amount of hydroxides and oxides, even when ground samples are used. In general, the lower the acid concentration the higher the amount of hydroxides/oxides formed on the metal surface. This observation is related to the possible formation of magnesium hydroxide during the HF treatment in aqueous solutions. Magnesium hydroxide can be formed by the reaction of the metal with water (Eqn (1.2)), which is the main reaction in the aqueous corrosion of magnesium. By comparing Eqns (1.1) and (1.2) it can be seen that both reactions have a similar thermodynamic tendency to take place. Therefore, from a thermodynamic point of view, it is expected that these reactions occur simultaneously when metallic magnesium is in contact with water and HF. The rate of each process will depend on the HF concentration.
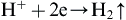
Increasing the acid concentration assures a coating constituted basically...
Table of contents
- Cover image
- Title page
- Table of Contents
- Related titles
- Copyright
- List of contributors
- Woodhead Publishing Series in Biomaterials
- Part One. Chemical and physical modifications of magnesium and its alloys for biomedical applications
- Part Two. Mechanical and electrochemical modifications of magnesium and its alloys for biomedical applications
- Part Three. Biomimetic modifications, surface functionalization of biomolecules, natural, conducting and biodegradable polymeric coatings
- Part Four. Other methods of surface modification of magnesium and its alloys
- Index