
Biomaterials in Plastic Surgery
Breast Implants
- 240 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About this book
Employed for both cosmetic and reconstructive purposes, breast implants are one of the most widely-used and controversial prostheses available. The development of safe, reliable products is vital to the future of this important field of surgery. Biomaterials in plastic surgery reviews the history, materials and safety issues associated with breast implants.Beginning with an introduction to the history of biomaterials used for breast augmentation, Biomaterials in plastic surgery goes on to discuss development issues. It then discusses the chemistry and physical properties of biomedical silicones before reviewing cohesive gel and polyurethane foam implants. The book concludes by analysing the epidemiological evidence on the safety issues relating to breast implants, followed by a review of retrieval and analysis of breast implants emphasizing strength, durability and failure mechanisms.With its distinguished editors and international team of expert contributors, Biomaterials in plastic surgery is an important guide for surgeons, manufacturers and all those researching this important field.- Comprehensively examines the history, materials and safety issues associated with breast implants- Provides an overview of the history of biomaterials used for breast augmentation and goes on to discuss the development and chemical and physical properties of biomedical silicones- Reviews cohesive gel breast implants and polyurethane foam breast implants
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
The history of biomaterials used for breast augmentation
Abstract:
1.1 Introduction
| • | Injectable materials | 1899–2010 |
| • | Sponges | 1899–2010 |
| • | Breast implants | 1963–2010 |
1.2 Injectable materials
| • | Paraffin | 1899–1914 |
| • | Other materials | 1915–1943 |
| • | Liquid silicone | 1944–1991 |
| • | Polyacrylamide hydrogel (PAH) | 1988–2009 |
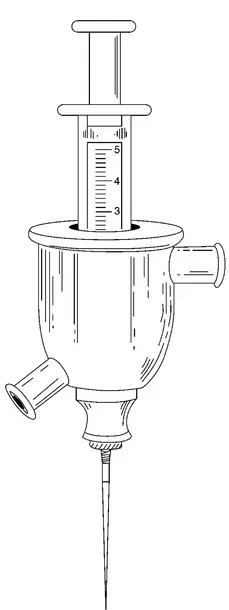
1.2.1 Paraffin, 1899–1914
1.2.2 Other injectable materials, 1915–1943
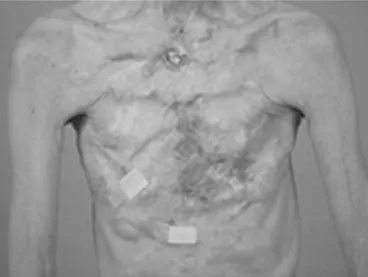
1.2.3 Liquid silicone injections, 1944–1991
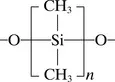
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Contributor contact details
- Woodhead Publishing Series in Biomaterials
- Introduction
- Chapter 1: The history of biomaterials used for breast augmentation
- Chapter 2: The development of breast implants
- Chapter 3: The chemistry and physical properties of biomedical silicones
- Chapter 4: Cohesive gel breast implants
- Chapter 5: Polyurethane foam covered breast implants
- Chapter 6: The safety of breast implants: epidemiologic studies
- Chapter 7: Retrieval and analysis of breast implants emphasizing strength, durability, and failure mechanisms
- Index