
- 362 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Introduction to Neuropharmacology
About this book
Introduction to Neuropharmacology presents the action of drugs as it relates to nervous system. It discusses the purposes into which drugs are use (e.g. as contraceptives and anti-riot agents). It addresses the differences between physiology and pharmacology. Some of the topics covered in the book are the factors affecting responses to drugs; properties of drugs; the kinetics of drug-receptor interactions; dose-response relationship; the principles of synaptic transmission; criteria for synaptic transmitters; somatic motor system; drugs affecting neuromuscular transmission; and drugs which act post-synaptically. The venoms and toxins that affect neuromuscular transmission are covered. The subdivisions of the autonomic nervous system are discussed. The text describes the autonomic ganglion stimulants. A study of the drugs mimicking parasympathetic stimulation is presented. A chapter is devoted to the drugs with antagonist actions on adrenoceptors. Another section focuses on the clinical uses of local anaesthetic drugs and the neurotransmitters in the central nervous system. The book can provide useful information to dentists, doctors, pharmacists, neurologists, students, and researchers.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Introduction to Neuropharmacology by Philip B. Bradley in PDF and/or ePUB format, as well as other popular books in Medicine & Neurology. We have over one million books available in our catalogue for you to explore.
Information
Part 1
General principles
Outline
Chapter 1: Characteristics of drug action
Chapter 2: Factors affecting responses to drugs
Chapter 3: The principles of synaptic transmission
Chapter 1
Characteristics of drug action
Publisher Summary
This chapter describes various characteristics of drug action. There are two important features concerning the biological action of drugs: one is that most drugs produce their effects in very small doses—that is, in very low concentrations in the tissues, and the other is that the majority of drugs are highly specific in terms of their chemical structure. The high potency of drugs can, therefore, be explained in terms of an interaction between the molecules of the drug and a specialized region of the cell membrane, the receptor, which mediates the response of the cell. The chemical specificity of drugs is explained in terms of a chemical relationship between the drug molecule and the receptor. The biological action of a drug is, therefore, explained in terms of an interaction with specific receptors for that drug. A drug that produces a response is known as an agonist, and agonists possess all three of the properties—namely, selectivity, affinity, and intrinsic activity.
There are two important features concerning the biological action of drugs. The first is that most drugs produce their effects in very small doses, i.e. in very low concentrations in the tissues. Thus, if it were possible to calculate the number of molecules of a drug present in a tissue, and to try to relate this to the surface area of the cells on which the drug was presumably acting, then it would be found that there were not sufficient molecules of the drug present to cover even a fraction of the total cell surface. How then does the drug modify the function of the cells?
The second feature is that the majority of drugs are highly specific in terms of their chemical structure. Thus, altering the structure of a drug may result in a reduction in, or a complete loss of, biological activity and there are many examples of stereoselectivity where only one isomer or enantiomorph is pharmacologically active. To explain these two features of the actions of drugs, the existence of a specialized region of the cell membrane, or ‘receptor’, is postulated and it is with this that the drug interacts. The receptor concept is usually attributed to a German chemist, Paul Erhlich (1900), who proposed the term ‘receptive substance’ for the chemical groups in the tissue which produced a biological response by combining with complementary groups of ‘foreign’ molecules, i.e. drugs. A similar idea was put forward by the English physiologist Langley (1878), to explain the actions of pilocarpine and atropine on salivary secretion.
The high potency of drugs can therefore be explained in terms of an interaction between the molecules of the drug and a specialized region of the cell membrane, the receptor, which mediates the response of the cell. The chemical specificity of drugs is explained in terms of a chemical relationship between the drug molecule and the receptor. The receptor concept is of fundamental importance to pharmacology, which would have no rational basis without it.
The biological action of a drug is therefore explained in terms of an interaction with specific receptors for that drug. This interaction is thought to be a physicochemical reaction which depends on the molecules of the drug being attracted to a corresponding or complementary molecular structure of the receptor. In order to explain this in more detail, drugs can be considered to possess three properties: selectivity, affinity and intrinsic activity.
Selectivity
This relates to the specificity of a drug for a particular receptor. A drug which is selective, and not all drugs are, will interact only with its own receptors and not those for other drugs or other types of drug. In this way a drug will produce effects in tissues or organs where its receptors are present and not where they are absent, and we can explain why drugs act only at certain sites in terms of the presence of its specific receptors and the selectivity of the drug for those receptors. A very simple analogy for this is that of the lock (receptor) and key (drug), but a better analogy is illustrated diagrammatically in Figure 1.1a, where the drug is seen to have the correct ‘shape’ to fit the receptor.
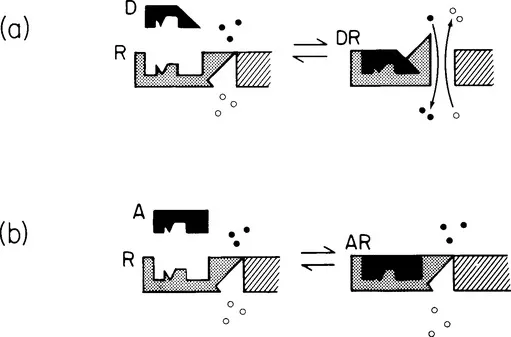
Figure 1.1 Diagrammatic representation of drug-receptor interaction. (a) Drug D has the correct shape to fit the receptor R, forming a drug-receptor complex DR and this results in a conformational change in the receptor and the opening of a pore in the adjacent membrane. Drug D is therefore an agonist. In (b) drug A also has the correct shape to fit the receptor, forming a drug-receptor complex AR, but in this case there is no conformational change, i.e. no response. Drug A is therefore an antagonist
Affinity
Apart from the drug having the right shape or chemical structure to fit the receptor, there must also be some force or forces attracting the molecules of the drug to the surface of the receptor in order to form a drug-receptor complex, which initiates the biological response. This attraction of the drug for the receptor is known as the ‘affinity’ of the drug and, because most drug-receptor interactions are reversible, the forces involved are normally weak chemical bonds, of which there are three main types, as follows:
1. Ionic bonds represent the electrostatic attraction between oppositely charged ions. Drug molecules are often large and contain many potential cationic and anionic groups of all kinds, capable of forming ionic bonds with oppositely charged groups on the receptor. The proteins and nucleic acids of the receptor will also possess potential cationic and anionic groups. In addition, many drugs are ionized in solution and will therefore be charged. Ionic bonds dissociate readily, so the drug-receptor interactions in which they are involved will be reversible. The bond strength is of the order of 21 kJ mol–1 (5 kcal mol–1) and the force of attraction (F) between the ionic groups diminishes as the square of the distance (r) between them, i.e. F∝ l/r2.
2. Hydrogen bonds represent a special kind of ionic bond. Many hydrogen atoms, present on or near the surface of a molecule—particularly a large molecule—possess a partial positive charge and can therefore form ionic bonds with negatively charged atoms, such as oxygen or nitrogen. Hydrogen bonds are usually weaker than ionic bonds, the bond strength being of the order of 8–21 kJ mol–1 (2–5 kcal mol–1) and inversely proportional to the fourth power of the distance between the drug and the receptor, i.e. F∝ l/r4.
3. van der Waals′ bonds or dipole bonds, are formed by the weak attraction between dipoles or induced dipoles, arising from the distortion of the orbits of outer electrons of atoms when the latter are in close proximity to one another. They are weak, the bond strength being of the order of 2 kJ mol–1 (0.5 kcal mol–1), and decrease in strength even more rapidly with increasing separation, i.e. F∝ 1/r7.
It is probable that all three types of reversible bond, i.e. ionic, hydrogen and van der Waals’, have a role in drug-receptor interactions. Thus, ionic bonding may be important initially in attracting the molecules of the drug to the surface of the receptor, but as the two come closer together, hydrogen and van der Waals’ forces may come into play. The affinity of a drug for its receptor may therefore depend upon the presence of a number of bonds of different types. In addition, van der Waals’ bonds, although relatively weak, when summed up over a large number of individual atoms may result in considerable binding strength. Furthermore, the combination of a number of bonds of different types over the surface of the drug molecule and its receptor probably helps to determine the degree of ‘fit’ which is achieved, and also helps to determine the degree of selectivity of the drug. This is illustrated for the cholinergic receptor in 4.6 (see Chapter 4).
There is a fourth type of chemical bond which needs to be considered as it does occur in pharmacology, although rarely. This is the covalent bond which is formed when two atoms share a pair of electrons. It is the bond which holds together the atoms of organic molecules and is relatively strong, with a bond strengt...
Table of contents
- Cover image
- Title page
- Table of Contents
- Dedication
- Copyright
- Preface
- Introduction
- Part 1: General principles
- Part 2: The peripheral nervous system
- Part 3: The central nervous system
- References to sources of illustrations
- Further reading
- Index