
- 432 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Lead Compounds from Medicinal Plants for the Treatment of Cancer
About this book
Lead Compounds from Medicinal Plants for the Treatment of Cancer is the first volume in the series, Pharmaceutical Leads from Medicinal Plants. The plant species described in this reference have been carefully selected based on pharmacological evidence and represent today's most promising sources of natural products for the discovery of anti-cancer drugs. Containing references to primary source material, over a hundred botanical illustrations, a table of chemical structures and much more, this book is an essential starting point for cancer researchers and those involved in anti-cancer drug discovery helping you identify the best novel lead molecules for further anti-cancer drug development.
- Provides a compilation of hundreds of medicinal plants from Europe, Asia, North and South America and Africa that contain prominent lead candidates for anti-cancer drug discovery
- Contains primary source references and hundreds of the most relevant citations from the current literature for additional research
- Offers cancer researchers and pharmaceutical scientists valuable tools such as chemical structures and promising pharmacological data to help them select the novel lead compounds that will best aid drug discovery.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Lead Compounds from Medicinal Plants for the Treatment of Cancer by Christophe Wiart in PDF and/or ePUB format, as well as other popular books in Medicine & Pharmacology. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
Alkaloids
Introduction
Caspases are proteolytic cysteine proteases that mediate apoptosis. One such cysteine protease is caspase 3, which is involved with the apoptosis induced by alkaloids such as brucine from Strychnos nux-vomica L. (1.2.5), tetrandrine from Stephania tetrandra S. Moore (1.3.4), anonaine from Michelia alba DC. (1.3.7) and sanguinarine from Macleaya microcarpa (Maxim.) Fedde (1.3.8). During apoptosis caspase 3 is activated by cytochrome c, which is released in the cytoplasm by insulted mitochondria via either the ‘intrinsic’ and/or ‘extrinsic’ pathway. Several alkaloids induce apoptosis via the intrinsic pathway, which involves the activation of ‘wild-type’ pro-apoptotic protein (p53), resulting in the stimulation of pro-apoptotic Bcl-2-associated X protein (Bax)-induced mitochondrial insult and consequent leakage of cytochrome c. Berberine from Corydalis ternata (Nakai) Nakai (1.3.5), tetrandrine from Stephania tetrandra S. Moore (1.3.4) and liriodenine from Goniothalamus amuyon (Blco.) Merr (1.3.7) activate pro-apoptotic protein (p53). The pro-apoptotic protein (p53) is controlled by a protein kinase (Akt), which has an immense influence on cell survival. Interestingly, alkaloids such as sauristolactam from Saururus chinensis (Lour) Baill. (1.3.3) or tetrandrine (1.3.4) inhibit Akt. The extrinsic pathway involves the stimulation of death receptors such as TNF-α receptor and Fas by extracellular messengers. Activation of death receptors is followed by the activation of caspase 8, which incurs mitochondrial insult via the cleavage of Bid (BH3 interacting-domain death agonist) and activates caspase 3. The alkaloids 6-hydroxythiobinupharidine, 6,6′-hydroxythiobinupharidine and 6-hydroxythionuphlutine B activate caspase 8 (1.4.2).
Other cellular mechanisms that induce apoptosis by targeting nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) involve cyclins, topoisomerase II, reactive oxygen species (ROS) and Ca2+ homeostasis, and microtubules. Note that piperine from Piper nigrum L. (1.1.3), 6-hydroxythiobinupharidine and 6-hydroxythiobinuphlutine B (1.4.2) and tylophorinidine from Tylophora atrofolliculata F.P. Metcalf (1.5.2) inhibits NF-κB and abrogates the survival of malignant cells. Berberine (1.3.1) inhibits cyclin B and cyclin-dependent kinase 1 (CDK1), the aristolactam SCH 546909 from Aristolochia manshuriensis Kom. (1.3.3) inhibits cyclin-dependent kinase 2 (CDK2) and tylophorine from Tylophora indica (Burm. f.) Merr. (1.5.2) inhibits cyclin A2, hence blockage of mitosis. Planar alkaloids such as ellipticine from Ochrosia elliptica Labill. (1.2.2), olivacine from Aspidosperma australe Müll. Arg. (1.2.2) and liriodenine (1.3.7) have the tendency to intercalate into DNA and inhibit the enzymatic activity of topoisomerase II, leading to DNA damage. Sanguinarine from Sanguinaria canadensis L. (1.3.8), jatrorrhizine (1.3.1) and tetrandrine (1.3.4) boost the cytoplasmic levels of ROS, whereas geissoschizine methyl ether and hirsutin from members of the genus Uncaria (1.2.3.) are Ca2+ blockers, and liriodenine (1.3.7) increases the cytoplasmic levels of nitric oxide (NO). Finally, alkaloids like colchicine, monoterpenoid indoles (1.2.1) and daphniglaucine C isolated from Daphniphyllum glaucescens Blume (1.4.1) inhibit the polymerization of tubulin and therefore abrogate the division of cancer cells.
In this chapter 1-[(9E)-10-(3,4-methylenedioxyphenyl)-9-decenoyl]pyrrolidine, N-cis-feruloyl tyramine, piplartine, guineensine, alstoyunine F, heptaphylline, vallesiachotamine, naucleaoral A, 12-methoxyechitamidine, alangiumkaloid A, SCH 546909, isotrilobine, demethylcorydalmine, racemosidine A, liriodenine, 6-methoxydihydrosanguinarine, daphniglaucine C, 6-hydroxythionuphlutine B, epipachysamine E, 2-(2-bromophenyl)-5,6,7-trihydroxy-8-((2-hydroxy-4-methylpiperazin-1-yl) methyl)- 4H-chromen-4-one and phenanthroindolizidine and their derivatives are identified as lead alkaloids for the treatment of cancer.
Topic 1.1
Amide Alkaloids
1.1.1 Piper Boehmeriifolium (Miq.) Wall. ex C. DC.
History
The plant was first described by Nathaniel Wallich in Prodromus Systematis Naturalis Regni Vegetabilis, published in 1869.
Synonym
Chavica boehmeriifolia Miquel
Family
Piperaceae Giseke, 1792
Common Names
False-nettle-leaved pepper, chavya, (Sanskrit), zhu ye ju (Chinese)
Habitat and Description
This shrub grows wild in China, Bhutan, India, Malaysia, Myanmar, Thailand and Vietnam. The plant grows to 5 m high. The stems are terete smooth and glabrous. The leaves are simple. The petiole is 0.2 –1 cm long. The blade is oblong-lanceolate, 10 –25 cm × 4 –8 cm, membranaceous, asymmetrical at base oblique, acuminate at apex and exhibits 6–10 pairs of secondary nerves. The inflorescence is a spike which is 10 –20 cm long and opposite to the leaves. The fruits are drupaceous, globose and 0.3 cm in diameter (Figure 1.1).
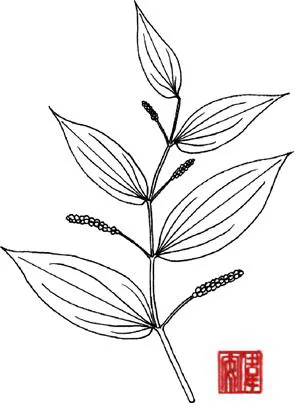
Figure 1.1 Piper boehmeriifolium (Miq.) Wall. ex C. DC.
Medicinal Uses
In China the whole plant is used to alleviate pain and for the treatment of rheumatism and arthritic conditions.
Phytopharmacology
Phytochemical investigations of Piper boehmeriifolium (Miq.) Wall. ex C. DC. resulted in the isolation of the amide alkaloid piperine,1 the aristolactam alkaloid piperolactam D2,3 and aporphine alkaloids.3 The anti-inflammatory and analgesic properties could be due to the isoquinoline contents of the plant since isoquinoline alkaloids suppress the receptor-mediated phospholipase A2 activation through uncoupling of a GTP binding protein from the enzyme.4
Proposed Research
Pharmacological study of 1-[(9E)-10-(3,4-methylenedioxyphenyl)-9-decenoyl]pyrrolidine for the treatment of cancer.
Rationale
In a recent study, Tang et al.5 isolated a series of amide alkaloids, one of which 1-[(9E)-10-(3,4-methylenedioxyphenyl)-9-decenoyl]pyrrolidine (CS 1.1) abrogated the survival of human epitheloid cervix carcinoma (Hela) cells with an IC50 value of 2.7 μg/mL.5 The mode of action of this alkaloid is to date unknown but one could think of apoptosis. In fact, irniine (CS 1.2), a pyrrolidine alkaloid extracted from the tubers of Arisarum vulgare Targ. Tozz. (family Araceae Juss) induced important alterations of the nuclei, induced DNA and mitochondrial damage and oxidative...
Table of contents
- Cover image
- Title page
- Table of Contents
- Front-matter
- Copyright
- Foreword
- Foreword
- Preface
- About the Author
- Chapter 1. Alkaloids
- Chapter 2. Terpenes
- Chapter 3. Phenolics
- Index of Natural Products
- Index of Oncological and Related Terms
- Index of Plants
- Subject Index