![]()
AXONAL TRANSPORT
Outline
Chapter 10: NEURAL CONTROL OF GENE EXPRESSION OF SKELETAL MUSCLE FIBERS
Chapter 11: THE ROLE OF CALCIUM IN AXOPLASMIC TRANSPORT IN MAMMALIAN NERVE FIBERS
Chapter 12: AXONAL TRANSPORT AND INTRACELLULAR ORGANIZATION OF SKELETAL AND CONTRACTILE STRUCTURES IN MATURING NEURONS
Chapter 13: AXONAL TRANSPORT AND PERIPHERAL NERVE DISEASE IN MAN
Chapter 14: CHANGES IN AXONAL TRANSPORT IN VARIOUS EXPERIMENTAL NEUROPATHIES
Chapter 15: AXONAL TRANSPORT IN ACRYLAMIDE NEUROPATHY
Chapter 16: EXPERIMENTAL INVESTIGATION OF ALCOHOLIC NEUROPATHY
Chapter 18: CONDUCTANCES OF RAT SKELETAL MUSCLE FIBERS DURING RECOVERY FROM CHRONIC APPLICATION OF VINCRISTINE TO THE MOTOR NERVE
![]()
NEURAL CONTROL OF GENE EXPRESSION OF SKELETAL MUSCLE FIBERS
SALVATORE METAFORA1, ROBERTO COTRUFO2, ARMANDO FELSANI1, GIANFRANCO TAJANA3, BRUNO RUTIGLIANO4, ANTONIO DEL RIO1, PIER PAOLO DE PRISCO1, MARIA ROSARIA MONSURRO’2, MARINA MELONE2 and RICARDO MILEDI5, 1Laboratorio di Embriologia Molecolare, CNR; Arco Felice; Naples; ITALY; 2Clinica Neurologica (R), 1a Facolt à di Medicina e Chirurgia; Naples; 3Istituto di Anatomia Umana Normale; 2a Facoltà di Medicina e Chirurgia; Naples; 4Laboratorio Internazionale di Genetica e Biofisica, CNR; Naples; 5Department of Biophysics; University College; London; U.K.
ABSTRACT
Experimental results are presented in favour of the hypothesis that alpha. motoneurones control the gene expression of skeletal muscle; in fact, following denervation, we found significant changes in the complexity and molecular diversity of mRNA populations isolated from the ribosomal pellet of muscle homogenate, as compared with control muscles.
INTRODUCTION
Studies on the differentiation of slow and fast motor units and on the effects of denervation, direct and crossed reinnervation, block of nerve conduction, block of axonal flow, chronic electrical stimulations at various frequencies: on the properties of muscle fibers, have all led to the conclusion that alpha-motoneurones control some steps of differentiation and maintain the differentiated state of muscle fibers (1, 2).
Up to now, one of the main questions to be answered was how does the neural control operate at the muscular level. We have formulated the hypothesis that such control operates at the level of gene expression; an observation which suggested this hypothesis was that acetylcholine hypersensitivity of denervated muscles could be prevented by administration of Actinomycin D or Cicloheximide (1), drugs which inhibit the protein synthesis at the transcriptional and translational levels, respectively.
We have tried to give an answer to the above question by investigating the effects of denervation on the complexity and diversity of mRNA populations of a mammalian muscle. Our results indicate that, following denervation, there is a clear-cut change in the programme of muscle gene expression.
MATERIALS AND METHODS
All the procedures and the materials used can not be described here for lack of space and will be reported in the full paper to be published.
RESULTS AND DISCUSSION
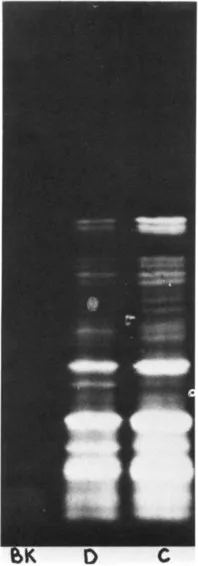
Following purification, by affinity chromatography on oligo-DT cellulose, of poly (A)+mRNA’s extracted from muscle ribosomal pellets, a big incubation of control or denervated poly(A)+mRNA’s in a mRNA dependent cell free protein synthesising system from wheat germ, in the presence of 35S-methionine, was performed. (3). Following incubation, the translation products were separated on slabs of polyacrylamide-SDS gels; from the observation of the autoradiography of these gels, several differences were found between the profile obtained following incubation with poly(A)+mRNA’s from control muscles and that obtained following incubation with poly(A)+mRNA’s from denervated muscles, as shown in figure 1. This result, indicating quantitative differences in some of the sequences present in mRNA populations isolated from denervated and contralateral muscles, suggested meaningful changes in the gene expression of skeletal muscles, following denervation.
Fig. 1 Autoradiography of the electrophoretic profiles, on slabs of SDS-polyacrylamide gels, of the translation products of the incubation of muscle poly(A)+mRNA’s in a mRNA-dependent, cell free protein synthesising system, from wheat germ, in the presence of 35S-methionine. Gels contained a continuous gradient of acrylamide (9% to 15%); the run was carried out from top to bottom of the figure; from top to bottom, the bands correspond to polypeptides of decreasing molecular weight. On the left side of the slab, was carried out the electrophoresis of the blank of incubation, containing all the constituents minus mRNA’s; no incorporation of 35S-methionine was found. In the middle, were separated the translation products of poly(A)+mRNA’s from denervated muscles; on the right side, the translation products of poly(A)+mRNA’s from control muscles. In all 3 runs, 50,000 cpm were deposited on the gel. The different proportions of the translation products of poly(A)+mRNA’s from control and denervated muscles, are self evident and indicate quantitative differences of some sequences in mRNA populations.
The poly(A)+mRNA’s, isolated from control and denervated muscles, were also used as templates to synthesize, by means of a reverse transcriptase, the relevant complementary DNA (cDNA). By using the radioactive cDNA thus obtained, we have kinetically studied the molecular hybridization between homologous poly(A)+mRNA and cDNA from control and denervated muscles, as shown in figures 2 and 3. The mathematical elaboration of the kinetic curves (4,5,6), reported in tables 1 and 2 for control and denervated mRNA populations, respectively, showed that, in control mRNA populations, four different classes of abundance were present (Table 1), while, in denervated mRNA populations, only three classes of abundance were detectable (Table 2). In particular, the first class of abundance found in control mRNA populations (whose complexity was equal to 2×106 daltons), including 3 different sequences of 700,000 daltons, each repeated 3,200 times per diploid genome (see also Table 1), was not present as a single class in denervated mRNA populations (table 2); therefore the most abundant
TABLE 1
COMPLEXITY, DIVERSITY AND ABUNDANCE OF mRNA-POLY(A)+ POPULATION FROM CONTROL MUSCLE
Mathematical analysis of the hybridiz...