
eBook - ePub
Strategies to Modify the Drug Release from Pharmaceutical Systems
- 208 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Strategies to Modify the Drug Release from Pharmaceutical Systems
About this book
Since the earliest dosage forms to modern drug delivery systems, came a great development and growth of knowledge with respect to drug delivery. Strategies to Modify the Drug Release from Pharmaceutical Systems will address principles, systems, applications and advances in the field.It will be principally a textbook and a reference source of strategies to modify the drug release. Moreover, the characterization, mathematical and physicochemical models, applications and the systems will be discussed.- Addresses the principles, systems, applications and advances in the field of drug delivery- Highlightsthe mathematical and physicochemical principles related to strategies- Discussesdrug release and its possible modifications
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Strategies to Modify the Drug Release from Pharmaceutical Systems by Marcos Luciano Bruschi in PDF and/or ePUB format, as well as other popular books in Medicina & Industria farmacéutica, biotecnológica y sanitaria. We have over one million books available in our catalogue for you to explore.
Information
1
General considerations
Abstract
The dosage form needs to be correctly developed to provide proper administration for the patient to receive maximum benefit, and the conception of the therapeutic regimen is fundamental. Conventional therapeutic regimens are characterized by the use of large amounts of a drug, with a great fraction being excreted without exerting therapeutic effects. Alternative therapeutic regimens have been proposed to provide fast relief of symptoms and longer action of the drug, protecting the patient for more time and increasing the therapeutic compliance. To achieve these aims, changes in availability or bioavailability are necessary, without modifications of therapeutic activity of the drug. The dosage forms are each time more specialized, constituting true systems that should display characteristics to release the active agent at a rate that perfectly matches the real need in vivo for the duration of the therapy, and to deliver the drug exclusively to its target site.
Keywords
Drug
Therapeutic agent
Active agent
Formulation
Development
Pharmaceutics
Physicochemical properties
Biopharmacy
Routes of administration
Therapeutic regimens.
1.1 Pharmaceutics: Safety, quality, and efficacy
The origin of modern humans is from Africa and dates from approximately 100,000 years ago (Pena, 2002). Despite being relatively new, Homo sapiens possess a complex organism that originated from a unique cell. When the egg is fertilized, it divides into two cells that divide again, which result in four, and so on. Besides the division, these basic units of organisms differentiate and originate tissues, organs, and systems. Their equilibrium and homeostasis are very important to the body’s global health. However, diseases and disequilibrium are very common, and the constant evolution of science, aiming for cures and well-being, conduced to the utilization of the different kingdoms of nature.
Humans, with their intelligence, search constantly for new drugs and materials. The World Health Organization defines a drug as “any substance that is used to modify or explore physiological systems or pathological states for the benefit of the patient.” It is an active agent intended for use in diagnosis, mitigation, treatment, cure, or prevention (FDA, 1938).
The diversity of drug actions and effects on the body is very important and enables their selective use in the treatment of a range of common and rare conditions involving virtually every body organ, tissue, and cell (Allen, Popovich, & Ansel, 2011). However, adjuvants are necessary to make possible the administration of an active agent. Thus, the concept of formulation and dosage form is introduced when we observe that medicines rarely are composed just drugs.
Medicine, or formulated preparation, is defined as a pharmaceutical formulation containing one or more drugs, to be used in diagnosis, prevention and/or cure of diseases and their symptoms or the correction/modification of organic functions, either in human being or in animals. It is a means of administering active agents into the body in a safe, efficient, reproducible, and convenient manner.
Until the mid-nineteenth century, the production of drugs was essentially handmade, consisting of drugs from plant, mineral or animal origin. During the early twentieth century, scientific institutions focused on the implementation of the research and production of medicines, vaccines and serums. The beginning of fermentation and chemical synthesis as a technological process started in the 1930s. Between 1940 and 1960, the global pharmaceutical industry emerged with the launch of new products and changes in marketing strategies, influencing the prescription.
The thalidomide disaster led to more rigorous registration policies, clinical trials, and quality control of products, seeking the safety, efficacy, and quality of medicines. Thus, the accuracy required in the approval of drugs, demanding the accomplishing of expensive trials in humans, together with the gradual occupation of the vast majority of the market’s niche by innovations over time, have significantly raised the cost of developing a new drug. Figure 1.1 shows the steps to the process of medicine development. Many activities must be successfully completed at different points and in different dimensions along the critical path. They are performed for every product, and many of them are highly complex (FDA, 2004).
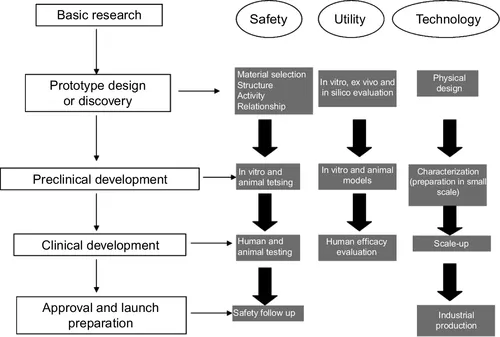
Figure 1.1 Generalized description of steps that must be successfully completed at different points and in different dimensions along the process of development and production of a medicine.
Pharmaceutical sciences are involved with the design, development, production, and use of medicines. Pharmaceutics is a scientific discipline of pharmacy that is about the conversion of a drug into a medicine suitable for administration by or to patients. The understanding of pharmaceutical preparation is very important, and the study of drug formulations and their design, manufacture, and delivery into the body is related to pharmaceutics (Florence & Attwood, 2006). Physical properties, dosage form design, and the manufacture of these medicines on both a small (compounding) and a large (pharmaceutical technology) scale are included in pharmaceutics (Aulton, 2001; Allen et al., 2011).
In the design of dosage forms, the physicochemical properties of the drug and biopharmaceutical and therapeutic considerations are very important:
What will be the administration route?
What is the disease to be treated?
What is the dose frequency for the drug?
The answers to these questions are very important to achieve the most suitable therapy. The medicine may vary from relatively simple solutions to complex drug delivery systems, using appropriate additives, excipients, or materials in the preparations. The aim is to achieve a better, more predictable therapeutic response to a drug included in a formulation, capable of being produced in large scale with reproducible quality.
To achieve this, microbiological, physical and chemical stability; precision of drug dose; packaging; labeling; and acceptability to users (prescriber and patient) are fundamental points that must be considered. Moreover, the natural variations between patients cannot interfere. This is difficult to achieve, but new knowledge, technologies and the vast array of drugs available today make it achievable. Nowadays, new active agents originated from animal, mineral or plant sources; chemical synthesis; molecular modification; biotechnology; or microbial technology are very important contributions for new medicines. Moreover, the evolution of computational technology has assisted the drug discovery, contributing to produce data banks and libraries of chemical compounds and methods of screening for potential biologic activity (Allen et al., 2011), and the obtaining of new dosage forms as well.
In this sense, for the development of new medicine, the first step is to know the chemical, physical, and biological characteristics of the active agent. The pharmacology of the drug and toxicological features must be determined. The drug’s site and rate of absorption, its pattern of distribution and concentration within the body, its duration of action, and the method and rate of its elimination or excretion must be studied (Allen et al., 2011).
Biopharmacy is a branch of pharmaceutical research that studies the physiological and pharmaceutical factors influencing drug release from the dosage form, its absorption by the body and bioavailability. The drug metabolism and the activity of any of its metabolites must be obtained. The short- and long-term effects of the active agent on body cells, tissues, and organs must be studied, as well as the drug’s ability to pass to a nursing baby through the mother’s breast milk, the hazardous effects of the drug on the fetus, and the complexation with plasmatic proteins.
Governmental agencies view the security and efficacy characteristics of new chemical entities each time more closely. The understanding of toxicological factors and metabolism of active agents is dependent on the patient age, drug distribution, genetic factors, time of exposition, and potential for teratogenic, mutagenic and embryotoxic effects. It is very important to investigate carefully to minimize possible toxic reactions, showing the safety and efficacy of new active agents.
The study of approved drugs conduces to the development of dosage forms with more efficacious delivery to the appropriate site, optimizing bioavailability, minimizing toxicity and side effects, and improving stability (Maurin, Hussain, & Dittert, 2006).
1.1.1 Routes of drug administration
Drugs can be introduced into the body by many routes, such as enteric (oral, peroral, rectal), parenteral (intravascular, intramuscular, subcutaneous, and inhalation administration) or topical (skin and mucosal membranes), among others, and must be determined considering the varying of individual ages (e.g., neonates, children, adults, geriatrics), weights and states of illness. Each route has specific purposes, advantages, and disadvantages (Table 1.1) (Allen et al., 2011; Aldridge, 2010).
Table 1.1
Main routes of medicine administration: advantages and disadvantages
| Route | Advantages | Disadvantages |
| Oral | Cheap, easy, no special equipment. Acceptable to most people. Suitable for self-medication | May be compromised by irritant effects/presence of food. Enzyme action may limit effectiveness |
| Sublingual | Drug absorption through buccal or sublingual mucosa avoids gut enzymes. Rapid action | Taste of drug may be a problem |
| Transdermal | Easy to use. Long action can be achieved. Avoids adverse effects of gastrointestinal tract enzymes | Relatively high cost. Drug may build up in skin so that action continues when patch is removed |
| Inhalation | Rapid action (inhaled anesthetics). Limits systemic absorption. Avoids gut enzymes | Needs specialized drug delivery system. Loss of dose—patient swallows most of the drug. Technique needs to be taught |
| Intranasal | Similar to inhalation | May irritate nasal mucosa. Needs special drug delivery system. Absorption may vary |
| Subcutaneous | Rapid absorption. Bypasses gastrointestinal tract. Patients may be taught to use this method | Absorption may be too rapid |
| Intramuscular | Good absorption. Bypasses gastrointestinal tract | Local irritancy. May be painful. Hazard of nerve damage. Skill involved |
| Intravenous | Rapid action can control rate of administration. Suitable for large volumes and drugs that would cause intramuscular irritation | Relatively high cost. Skill involved. Extravasation risk. Specialist drug delivery system... |
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Preface
- 1: General considerations
- 2: Modification of drug release
- 3: Classification of therapeutic systems for drug delivery
- 4: Main mechanisms to control the drug release
- 5: Mathematical models of drug release
- 6: Drug delivery systems
- Index