
- 608 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Polymer Nanocomposites
About this book
Polymer nanocomposites are polymer matrices reinforced with nano-scale fillers. This new class of composite materials has shown enhanced optical, electrical and dielectric properties. This important book begins by examining the characteristics of the main types of polymer nanocomposites and then reviews their diverse applications.Part one focuses on polymer/nanoparticle composites, their synthesis, optical properties and electrical conductivity. Part two describes the electrical, dielectric and thermal behaviour of polymer/nanoplatelet composites, whilst polymer/nanotube composites are the subject of Part three. The processing and industrial applications of these nanocomposite materials are discussed in Part four, including uses in fuel cells, bioimaging and sensors as well as the manufacture and applications of electrospun polymer nanocomposite fibers, nanostructured transition metal oxides, clay nanofiller/epoxy nanocomposites, hybrid epoxy-silica-rubber nanocomposites and other rubber-based nanocomposites.Polymer nanocomposites: physical properties and applications is a valuable reference tool for both the research community and industry professionals wanting to learn about these materials and their applications in such areas as fuel cell, sensor and biomedical technology.- Gives a comprehensive review of polymer nanocomposites and their properties- A standard reference on this area- Written by distinguished editors and a international team of authors
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Polyamide/clay nanocomposites
1.1 Introduction
1.2 Nylon 6-clay hybrid (NCH)
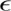
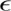
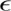
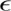
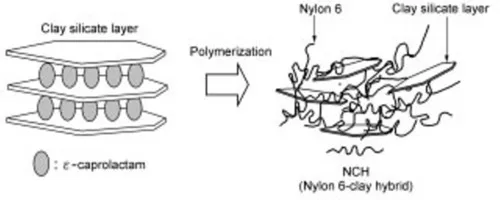
1.3 Synthesis of nylon 6-clay hybrid (NCH)
1.3.1 Clay organization and monomer swelling (Usuki, 1993a)
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright page
- Contributor contact details
- Preface
- Part I: Layered silicates
- Part II: Nanotubes, nanoparticles and inorganic-organic hybrid systems
- Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app