
- 1,216 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Encyclopedia of the Alkaline Earth Compounds
About this book
Encyclopedia of the Alkaline Earth Compounds is a compilation describing the physical and chemical properties of all of the alkaline earth compounds that have been elucidated to date in the scientific literature. These compounds are used in applications such as LEDs and electronic devices such as smart phones and tablet computers. Preparation methods for each compound are presented to show which techniques have been successful. Structures and phase diagrams are presented where applicable to aid in understanding the complexities of the topics discussed.
With concise descriptions presenting the chemical, physical and electrical properties of any given compound, this subject matter will serve as an introduction to the field. This compendium is vital for students and scientific researchers in all fields of scientific endeavors, including non-chemists.
- 2013 Honorable Mention in Chemistry & Physics from the Association of American Publishers' PROSE Awards
- Presents a systematic coverage of all known alkaline earth inorganic compounds and their properties
- Provides a clear, consistent presentation based on groups facilitatating easy comparisons
- Includes the structure of all the compounds in high quality full-color graphics
- Summarizes all currently known properties of the transition metals compounds
- Lists the uses and applications of these compounds in electronics, energy, and catalysis
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Encyclopedia of the Alkaline Earth Compounds by Richard C. Ropp in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Inorganic Chemistry. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
The Alkaline Earths as Metals
Outline
1.1. General Properties
1.2. Properties of the Alkaline Earth Metals
1.2.1. Beryllium
1.2.2. Magnesium
1.2.3. Calcium
1.2.4. Strontium
1.2.5. Barium
1.2.6. Radium
The alkaline earth metals comprise Group 2 of the periodic table and include the elements Be, Mg, Ca, Sr, Ba and Ra. These metals form cations with a formal charge of +2 in solution and are the second most electropositive metals of all of the elements (the alkali metals are the most electropositive). The name of this specific group in the periodic table stems from the fact that their oxides produce basic alkaline solutions and that these elements melt at such high temperatures that they remain solid (earths) in fires. The alkaline earth metals provide a good example of group trends in chemical properties within the periodic table, with well-characterized homologous behavior as one goes down the group. With the exception of Be and Mg, the metals have a distinguishable flame color, orange-red for Ca, magenta-red for Sr, green for Ba and crimson-red for Ra.
1.1 General Properties
Like other groups, the members of this family show specific patterns in their electron configuration, especially the outermost shells, that results in trends in chemical behavior (Table 1.1).
TABLE 1.1
| Z | Element | No. of electrons/shell |
| 4 | Beryllium | 2, 2 |
| 12 | Magnesium | 2, 8, 2 |
| 20 | Calcium | 2, 8, 8, 2 |
| 38 | Strontium | 2, 8, 18, 8, 2 |
| 56 | Barium | 2, 8, 18, 18, 8, 2 |
| 88 | Radium | 2, 8, 18, 32, 18, 8, 2 |
Another way to depict the electronic structure of these elements is shown in Table 1.2.
TABLE 1.2
| Element | Symbol | Electronic configuration |
| Beryllium | Be | [He]2s2 |
| Magnesium | Mg | [Ne]3s2 |
| Calcium | Ca | [Ar]4s2 |
| Strontium | Sr | [Kr]5s2 |
| Barium | Ba | [Xe]6s2 |
| Radium | Ra | [Rn]7s2 |
All of the alkaline earth metals have two electrons in their outer valence shell, so the energetically preferred state of achieving a filled electron shell is to lose two electrons to form doubly charged cations, M2+. The alkaline earth metals are silver-colored, soft metals that react readily with halogens to form ionic salts. They also react with water, though not as rapidly as the alkali metals, to form strongly alkaline (basic) hydroxides. For example, whereas Na and K react with water at room temperature, Mg reacts only with steam and Ca with hot water:
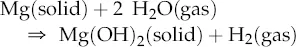
Be is an exception. It does not react with water or steam, and its halides are covalent.
The alkaline earth metals are named after their oxides, the alkaline earths, whose old-fashioned names were Beryllia, Magnesia, Lime, Strontia and Baryta. “Earth” is the old term applied by early chemists to nonmetallic substances that were insoluble in water and resistant to heating, properties shared by these oxides. The realization that these earths were not elements but compounds is attributed to the chemist Antoine Lavoisier. In his “Traité Élementaire de Chemie” (Elements of Chemistry) of 1789, he called them “salt-forming” earth elements. Later, he suggested that the alkaline earths might be metal oxides, but admitted that this was mere conjecture. In 1808, acting on Lavoisier’s idea, Humphrey Davy became the first to obtain samples of the metals by electrolysis of their molten “earths”.
If the alkaline earths are compared to the alkalis, many similarities are apparent. The main difference is the electron configuration, which is ns2 for alkaline earth metals and ns1 for alkali metals. But for the alkaline earth metals, the nucleus also contains an additional positive charge. Also, the elements of Group 2 (alkaline earths) have much higher melting points and boiling points compared to those of Group 1 (alkali metals). The alkalis are softer and more lightweight than the alkaline earth metals that are much harder and denser.
The second valence electron is very important when it comes to comparing chemical properties of the alkaline earth and the alkali metals. The second valence electron is in the same “sublevel” as the first valence electron. Therefore, the Zeff is much greater. This means that the elements of Group 2 have a smaller atomic radius and much higher ionization energy than those of Group 1. Even though the Group 2 contains a much higher ionization energy, they still form ionic compounds containing 2+ cations. Beryllium, however, behaves differently. This is due to the fact that in order to remove two electrons from this particular atom, significantly more energy is required. It never forms the Be2+ cation and its bonds are polar covalent.
Atomic and ionic radii increase smoothly down the Group. The ionic radii are all much smaller than the corresponding atomic radii. This arises because the atom contains two electrons in an s level relatively far from the nucleus. It is these electrons that are removed to form the ion. Remaining electrons are thus in levels closer to the nucleus, and in addition the increased effective nuclear charge attracts the electrons toward the nucleus and decreases the size of the ion.
These elements are all found in the Earth’s crust, but not in the elemental form because they are so reactive. Instead, they are widely distributed in rock structures. The main minerals in which magnesium is found are “Carnellite”, “Magnesite” and “Dolomite”. Calcium is found in “Chalk”, “Limestone”, “Gypsum” and “Anhydrite”. Magnesium is the eighth most abundant element in the Earth’s crust, and calcium is the fifth.
Some of the physical properties of the alkaline earth metals are shown in Table 1.3.
TABLE 1.3

The metals of Group 2 are harder and denser than sodium and potassium, and have higher melting points. These properties are due largely to the presence of two valence electrons on each atom, which leads to stronger metallic bonding than occurs in Group 1.
Three of these elements give characteristic colors when heated in a flame:
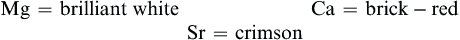
In all their compounds, these metals have an oxidation number of +2 and, with few exceptions, their compounds are ionic in nature. The reason for this can be seen by examination of the electron configuration, which always has two electrons in an outer quantum level. These electrons are relatively easy to remove, but removing the third electron is much more difficult, as it is close to the nucleus and in a filled quantum shell. This results in the formation of M2+. The ionization energies reflect this electron arrangement. The first two ionization energies are relatively low, and the third very much higher.
In general, the chemical properties of Group 2 elements are dominated by the strong reducing power of the metals. The elements become increasingly electropositive as one descends within the Group. In direct contact with oxygen or chlorine gas, little or no reaction occurs. However, once started, the reactions with oxygen and chlorine are vigorous:


All the metals except beryllium form oxide layers in air at room temperature that dulls the surface of the metal. Barium is so reactive that it is stored under oil. All of the metals except beryllium reduce water and dilute acids to hydrogen:
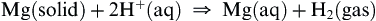
Magnesium reacts only slowly with water unless the water is boiling, but calcium reacts rapidly even at room temperature, and forms a cloudy white suspension of sparingly soluble calcium hydroxide.
Calcium, strontium and barium can reduce hydrogen gas when heated, forming the hydride:
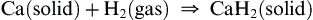
The hot metals are also sufficiently strong reducing agents to reduce nitrogen gas and form nitrides:
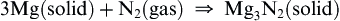
Magnesium can reduce, and burn, in carbon dioxide:

This means that magnesium fires cannot be extinguished using carbon dioxide fire extinguishers.
The oxides of alkaline earth metals are normally prepared by heating the hydroxide or carbonate to release carbon dioxide gas. They have high lattice enthalpies and melting points. Peroxides, MO2, are known for all these elements except beryllium. It appears that th...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Dedication
- Preface
- Chapter 1. The Alkaline Earths as Metals
- Chapter 2. Group 17 (H, F, Cl, Br, I) Alkaline Earth Compounds
- Chapter 3. Group 16 (O, S, Se, Te) Alkaline Earth Compounds
- Chapter 4. Group 15 (N, P, As, Sb and Bi) Alkaline Earth Compounds
- Chapter 5. Group 14 (C, Si, Ge, Sn, and Pb) Alkaline Earth Compounds
- Chapter 6. Group 13 (B, Al, Ga, In and Tl) Alkaline Earth Compounds
- Chapter 7. Group 3 (Sc, Y, and La) Alkaline Earth Compounds
- Chapter 8. Group 4 (Ti, Zr and Hf) Alkaline Earth Compounds
- Chapter 9. Group 5 (V, Nb and Ta) Alkaline Earth Compounds
- Chapter 10. Group 6 (Cr, Mo and W) Alkaline Earth Compounds
- Chapter 11. Group 7 (Mn, Tc and Re) Alkaline Earth Compounds
- Chapter 12. Group 8 (Fe, Ru and Os) Alkaline Earth Compounds
- Chapter 13. Group 9 (Co, Rh and Ir) Alkaline Earth Compounds
- Chapter 14. Group 10 (Ni, Pd and Pt) Alkaline Earth Compounds
- Chapter 15. Group 11 (Cu, Ag and Au) Alkaline Earth Compounds
- Chapter 16. Group 12 (Zn, Cd and Hg) Alkaline Earth Compounds
- General References
- Index