
- 412 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Asia is increasingly taking on a leading role in the fields of Good Clinical Practice (GCP) and ethics, two areas that are central to clinical research practices worldwide. Clinical research in Asia examines the evolution of these key sectors in the Asian countries where the greatest developments are taking place, offering valuable perspectives on a wide range of issues affecting clinical research. Following an introduction that provides an overview of the topic and its strengths and weaknesses, each chapter of the book is devoted to clinical research in a specific country, focusing on issues including the history and evolution of clinical research, clinical trials and regulatory aspects. The chapters also offer a perspective on future trends in clinical research in each country. The book concludes with a discussion of the importance of political, economic, socio-cultural, technological, legal and environmental factors (PESTLE analysis).
- Analysis from a leading and highly respected professional in the sector
- An overview of country-specific regulatory environments
- Discussion of challenges and solutions for clinical research
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Clinical Research in Asia by U Sahoo in PDF and/or ePUB format, as well as other popular books in Medicina & Farmacología. We have over one million books available in our catalogue for you to explore.
Information
1
Clinical research in Asia: a brief overview
Abstract:
Starting with a brief background of the drug development cycle, Chapter 1 delves into the global pharmaceutical market and key global pharmaceutical players in terms of market size and their clinical research initiatives. This chapter briefly discusses the clinical research business, the global market and key players and contract research organisations (CROs). It further narrows down to describe the clinical research boom and key players in Asia and undertakes a country segmentation analysis of Asia. This chapter summarises the number of trials undertaken in Asia, inspections and audits performed in Asia and outlines a SWOT analysis.
1.1 Background
Clinical research forms the backbone of new product development in pharmaceutical business. It involves the testing of new drugs or new chemical entities for safety and efficacy in human beings and could pose risks to human life. It is always based on scientific rationale and highly regulated by government bodies. Clinical trials for a drug can cost up to $1 billion and can take as long as 12 years from discovery phase to marketing phase (Figure 1.1).
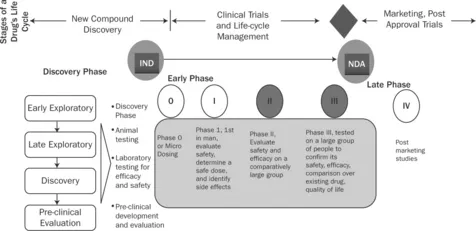
Figure 1.1 Drug development life-cycle
Until recently, most new pharmaceutical products were due largely to the sincere efforts and investments of the global pharma companies. Most of these companies have invested a lot of time and money to undertake clinical studies in the USA and Western Europe. Their success is due to increased public awareness for research and development, driven by relevant expertise among government bodies to implement regulations to protect the interests of human subjects. These regulations also safeguard the commercial interests of the pharma companies in terms of protecting their intellectual property.
1.2 Global pharmaceutical market
According to IMS Report, the global pharmaceutical market is presently worth $808 billion, and is estimated to grow at a compound annual growth rate (CAGR) of 5–8 per cent during 2009–14. The same analysis also forecasts that while developed markets such as the USA, Europe and Japan will grow at a CAGR of 2–6 per cent, the emerging countries of Asia, Africa, Australia and Latin America will grow at a CAGR of 12–15 per cent (Table 1.1).
Table 1.1
Global pharmaceutical market analysis
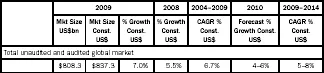
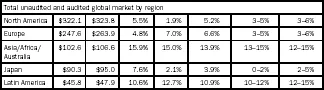
Source: IMS Health Market Prognosis, March 2010
Despite the similarity in environmental and disease traits, there are striking differences between the various Asian countries as far as pharmaceutical market size and health expenditure are concerned; this is primarily due to differences in regulatory and business environments. Table 1.2 highlights these differences across the Asian countries. Note that the Japanese and Chinese pharmaceutical markets are huge and hence attract significant investment.
Table 1.2
Pharmaceutical market size in select Asian countries
| Country | Market size ($) |
| Japan | 60bn |
| China | 20bn |
| India | 6bn |
| Taiwan | 2.5bn |
| Hong Kong | 1.5bn |
| Thailand | 1.5bn |
| Singapore | 400 m |
| Indonesia | 350 m |
| Philippines | 300 m |
| Malaysia | 210 m |
Source: World Bank, World Development Indicators 2006
Again, per capita health expenditure between different countries in Asia varies significantly, from $27 in India to $2,662 in Japan (Table 1.3). Considering the huge population base in Asian countries, the region offers huge value propositions for pharmaceutical companies to undertake clinical trials and market their products in Asian countries.
Table 1.3
Health expenditure in select Asian countries
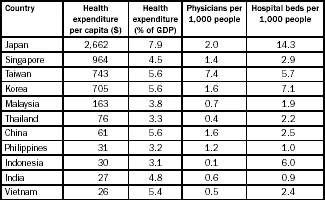
Source: World Bank, World Development Indicators 2006
Global pharmaceutical players contribute significantly in providing healthcare medicine for mankind. From Table 1.4 it is evident that the main multinational pharma companies have a robust product portfolio that yields a huge sales revenue. These companies invest large sums of money in research and development. Their objective is to develop new drugs and devices to fight advanced chronic or rare diseases. In the process, pharmaceutical and biotech companies undertake a number of trials to prove not only that their products are safe, but also that they are more efficacious than what exists in the market already. These trials are undertaken in preclinical and clinical phases from Phase I to IV, which is time-consuming and very cost-intensive. As shown in Table 1.4, these 15 global pharmaceutical players were und...
Table of contents
- Cover image
- Title page
- Table of Contents
- Dedication
- Copyright
- List of figures and table
- Acknowledgments
- List of abbreviations
- Preface
- About the author
- Chapter 1: Clinical research in Asia: a brief overview
- Chapter 2: Clinical research in Japan
- Chapter 3: Clinical research in India
- Chapter 4: Clinical research in China
- Chapter 5: Clinical research in South Korea
- Chapter 6: Clinical research in Taiwan
- Chapter 7: Clinical research in Singapore
- Chapter 8: Clinical research in Thailand
- Chapter 9: Clinical research in Malaysia
- Chapter 10: Clinical research in Hong Kong
- Chapter 11: Clinical research in the Philippines, Indonesia Vietnam
- Chapter 12: Conclusions
- Bibliography
- Index