
- 656 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Corrosion of Magnesium Alloys
About this book
The use of magnesium alloys is increasing in a range of applications, and their popularity is growing wherever lightweight materials are needed. This book provides a comprehensive account of the corrosion of magnesium alloys. It covers not only the corrosion performances and mechanisms of Mg alloys in conventional environments, such as sodium chloride solutions, but also looks at their corrosion behaviours in special media, like engine coolants and simulated body fluids.Part one covers fundamentals such as the corrosion electrochemistry, activity and passivity of magnesium and its alloys. Part two then considers the metallurgical effect in relation to the corrosion of magnesium alloys, including the role of micro-structure and earth-rare elements, the corrosion behaviour of magnesium-based bulk metallic glasses, and the corrosion of innovative magnesium alloys. Part three goes on to describe environmental influences on the corrosion of magnesium alloys, such as atmospheric corrosion, stress corrosion cracking, creep and fatigue behaviour, and galvanic corrosion. Finally, part four is concerned with various means of protecting magnesium alloys against corrosion through the use of aluminium electrodeposition, conversion and electrophoretic coatings, and anodisation.With its distinguished editor and team of contributors, this book is an invaluable resource for metallurgists, engineers and designers working with magnesium and its alloys, as well as professionals in the aerospace and automotive industries.- Provides a comprehensive account of the corrosion of magnesium alloys covering fundamentals such as the corrosion electrochemistry, activity and passivity- Reviews the metallurgical effect in relation to the corrosion of magnesium alloys, including the role of micro-structure and earth-rare elements- Assesses environmental influences such as atmospheric corrosion, stress corrosion cracking, creep and fatigue behaviour, and galvanic corrosion
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Corrosion electrochemistry of magnesium (Mg) and its alloys
Abstract:
1.1 Introduction
1.2 Thermodynamics
1.2.1 Thermodynamic tendency
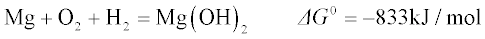

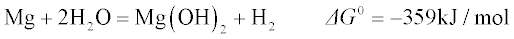
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Contributor contact details
- Preface
- Part I: Fundamentals
- Part II: Metallurgical effects
- Part III: Environmental influences
- Part IV: Corrosion protection
- Index