
- 458 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Carbon Nanotubes and Graphene
About this book
Carbon Nanotubes and Graphene is a timely second edition of the original Science and Technology of Carbon Nanotubes. Updated to include expanded coverage of the preparation, purification, structural characterization, and common application areas of single- and multi-walled CNT structures, this work compares, contrasts, and, where appropriate, unitizes CNT to graphene. This much expanded second edition reference supports knowledge discovery, production of impactful carbon research, encourages transition between research fields, and aids the formation of emergent applications. New chapters encompass recent developments in the theoretical treatments of electronic and vibrational structures, and magnetic, optical, and electrical solid-state properties, providing a vital base to research. Current and potential applications of both materials, including the prospect for large-scale synthesis of graphene, biological structures, and flexible electronics, are also critically discussed.
- Updated discussion of properties, structure, and morphology of biological and flexible electronic applications aids fundamental knowledge discovery
- Innovative parallel focus on nanotubes and graphene enables you to learn from the successes and failures of, respectively, mature and emergent partner research disciplines
- High-quality figures and tables on physical and mathematical applications expertly summarize key information – essential if you need quick, critically relevant data
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Carbon Nanotubes and Graphene by Kazuyoshi Tanaka,S. Iijima in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Physical & Theoretical Chemistry. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
Classification of Carbon
Kazuyoshi Tanaka Department of Molecular Engineering, Kyoto University, Nishikyo-ku, Kyoto 615-8510, Japan
Abstract
A simple classification of carbon material based on the hybridization state and [H]/[C] atomic ratio is summarized. It is noted that π conjugation of carbon materials is rather robust, which appears on the curved surface of fullerene and carbon nanotubes.
Keywords
spn-hybridization
[H]/[C] atomic ratio
graphene
graphite
fullerene
carbon nanotube (CNT)
amorphous carbon (a-C)
carbine
diamond
One of the reasons of fertile chemical nature of carbon materials comes from its hybridization state, which also basically determines the features of the variety of organic compounds. In association with this fact, classification of carbon materials would be of importance, which can be simply seen in terms of a certain domain map as shown in Figure 1.1. In this map there are two axes: one is the degree of spn hybridization with taking n as variable and the other [H]/[C] atomic ratio of the material. Typical carbon materials and the relatives occupying specific points on this domain map are shown in Figure 1.2.
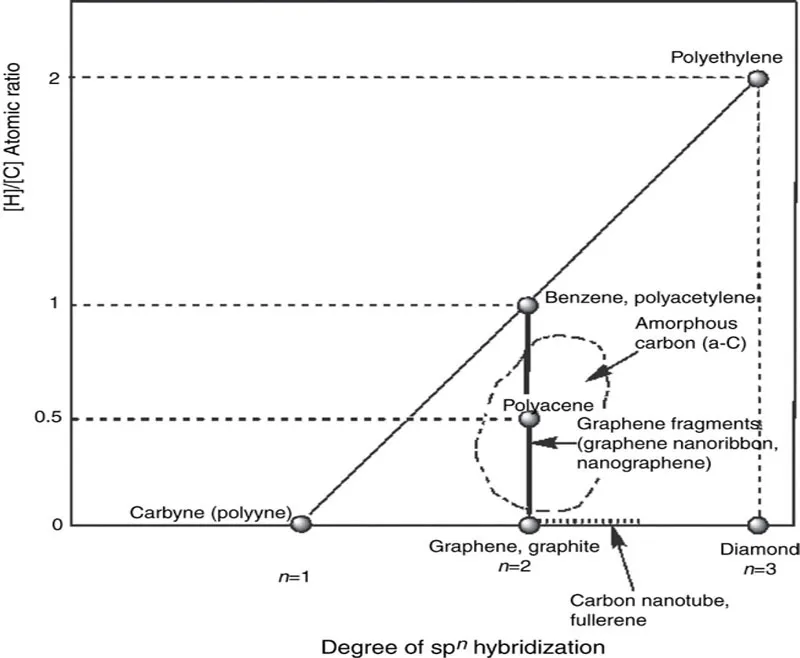
Figure 1.1 Domain map of carbon-material family.
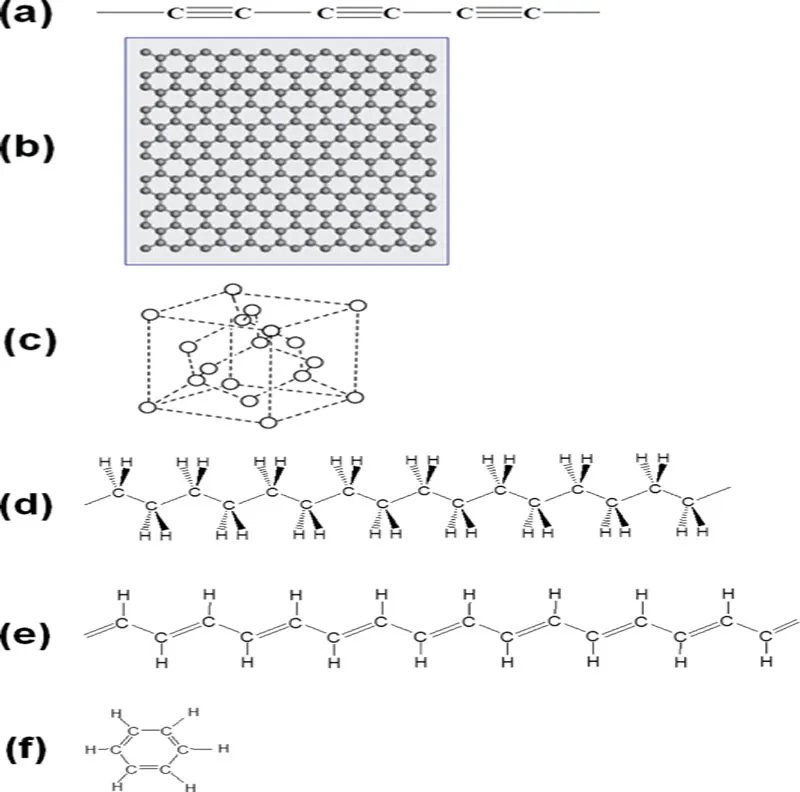
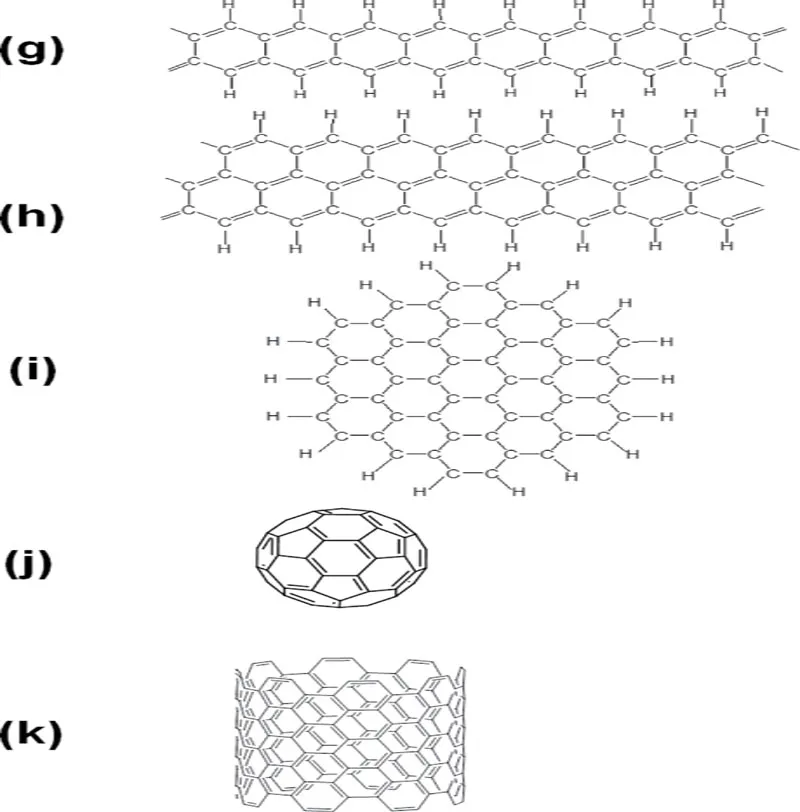
Figure 1.2 Various carbon materials and their relatives: (a) carbyne (polyyne), (b) graphene, (c) diamond, (d) polyethylene, (e) polyacetylene, (f) benzene, (g) polyacene, (h) graphene nanoribbon, (i) nanographene, (j) fullerene and (k) CNT.
Since graphene has complete sp2 hybridization at every carbon atom and hydrogen atoms are ideally not involved in the skeleton, it occupies the point (2, 0) on the map, being the same for graphite when neglecting the small interlayer interaction. On the other hand, the point (2, 1) for polyacetylene and benzene has complete sp2 hybridization with the [H]/[C] ratio of unity. On the line between the points (2, 1) and (2, 0) exists the graphene-fragments group including graphene nanoribbon and nanographene. Hence, the members belonging to this group also consist of sp2 hybridization but their [H]/[C] ratios are between 1 and 0 (mostly between 0.5 and 0) along with the development of carbon skeleton.
Fullerenes and carbon nanotubes (CNTs), moreover, belong to an interesting family without any hydrogen atoms but not have genuine sp2 hybridization due to the curved surfaces. In other words, they have the hybridization of sp2+ δ, a bit closer to sp3 (between n = 2 and 3) and, hence, they are on the broken line on the horizontal axis at the right to the point for graphene and graphite. It is, however, generally considered that these two materials are of π conjugation system in actuality. In this sense, π conjugation on the carbon atoms is rather robust even on the curved surface. This feature has been mentioned as σ-bond hybridization due to more mixing of 2s atomic orbital of carbon atom into the original 2pπ orbital [1].
Around the line of the graphene fragments is an ambiguous area surrounded by broken line called amorphous carbon (a-C) including coal, charcoal, coke, soot, carbon black and so on. These members are sometimes of importance in the industrial field and often consist of not only carbon and hydrogen atoms but also heteroatoms, such as oxygen, nitrogen or sulfur. Carbon fibres also belong to this group although they are rather near to graphite from the viewpoints of atomic arrangement. It is of interest to note that the precursor of vapour-grown carbon fibre (VGCF) ...
Table of contents
- Cover
- Title page
- Table of Contents
- Copyright
- List of Contributors
- Preface
- Chapter 1: Classification of Carbon
- Chapter 2: Multidimensional Aspects of Single-Wall Carbon Nanotube Synthesis
- Chapter 3: Differentiation of Carbon Nanotubes with Different Chirality
- Chapter 4: Preparation of Graphene with Large Area
- Chapter 5: Optical Properties of Carbon Nanotubes
- Chapter 6: Phonon Structures and Raman Effect of Carbon Nanotubes and Graphene
- Chapter 7: Transport Properties of Carbon Nanotubes and Graphene
- Chapter 8: Mechanical Properties of Carbon Nanotubes and Graphene
- Chapter 9: Organometallic Chemistry of Carbon Nanotubes and Graphene
- Chapter 10: Preparation and Properties of Carbon Nanopeapods
- Chapter 11: Applications of Carbon Nanotubes and Graphene in Spin Electronics
- Chapter 12: Biological Application of Carbon Nanotubes and Graphene
- Chapter 13: Characteristics and Applications of Carbon Nanotubes with Different Numbers of Walls
- Chapter 14: Graphene Oxide: Some New Insights into an Old Material
- Chapter 15: Graphene Nanoribbon and Nanographene
- Chapter 16: Application of Functional Hybrids Incorporating Carbon Nanotubes or Graphene
- Index