
- 424 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
High Resolution NMR provides a broad treatment of the principles and theory of nuclear magnetic resonance (NMR) as it is used in the chemical sciences. It is written at an "intermediate" level, with mathematics used to augment, rather than replace, clear verbal descriptions of the phenomena. The book is intended to allow a graduate student, advanced undergraduate, or researcher to understand NMR at a fundamental level, and to see illustrations of the applications of NMR to the determination of the structure of small organic molecules and macromolecules, including proteins. Emphasis is on the study of NMR in liquids, but the treatment also includes high resolution NMR in the solid state and the principles of NMR imaging and localized spectroscopy.
Careful attention is given to developing and interrelating four approaches - steady state energy levels, the rotating vector picture, the density matrix, and the product operator formalism. The presentation is based on the assumption that the reader has an acquaintance with the general principles of quantum mechanics, but no extensive background in quantum theory or proficiency in mathematics is required. Likewise, no previous background in NMR is assumed, since the book begins with a description of the basic physics, together with a brief account of the historical development of the field.
This third edition of High Resolution NMR preserves the "conversational" approach of the previous editions that has been well accepted as a teaching tool. However, more than half the material is new, and the remainder has been revised extensively. Problems are included to reinforce concepts in the book.
- Uses mathematics to augment, not replace, verbal explanations
- Written in a clear and conversational style
- Follows the successful format and approach of two previous editions
- Revised and updated extensively--about 70 percent of the text is new
- Includes problems and references to additional reading at the end of each chapter
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access High Resolution NMR by Edwin D. Becker in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Spectroscopy & Spectrum Analysis. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
Introduction
The first nuclear magnetic resonance (NMR) was detected early in 1938 in a molecular beam, and the first studies of NMR in bulk materials were carried out about 8 years later. Over the following decades, NMR has grown from an interesting and important study of a physical phenomenon to an indispensable technique in a very wide variety of fields. In organic chemistry NMR is arguably one of the two most important tools for the elucidation of molecular structure. In structural biology NMR rivals x-ray crystallography in providing precise three-dimensional structures for proteins and other macromolecules, but NMR goes beyond x-ray crystallography in furnishing information on internal mobility and overall molecular motion in both large and small molecules.
NMR has become one of the best methods for obtaining anatomical images of human subjects and animals (under the common name magnetic resonance imaging, MRI) and for exploring physiological processes. Materials science uses NMR spectroscopy and imaging to describe the structure, motion, and electronic properties of heterogeneous and technologically important substances. NMR is widely used in the food industry to measure moisture content and to assess the quality of certain foodstuffs. NMR is used to measure the flow of liquids in pipes in industrial processes and to observe the flow of blood in human beings. NMR is used in the exploration for petroleum, and it has even been used to search for submarines during wartime.
There is a vast literature on NMR and many books that describe all of these applications (and others). For example, the eight-volume Encyclopedia of Nuclear Magnetic Resonance describes a wide variety of NMR studies.1 Underlying all of these diverse applications, however, is a basic theory that treats the behavior of nuclear magnets in a magnetic field. In this book we cannot hope to provide a comprehensive treatment of NMR theory or application. Our intention, rather, is to explore the basic physical phenomena, primarily from the viewpoint of the chemist, to illustrate ways in which NMR spectroscopy can be applied in chemistry and structural biology and to point out very briefly how NMR imaging is applied in materials science and biomedicine. Although we use mathematics as needed, we try to provide an intuitive understanding insofar as possible, sometimes at the expense of mathematical rigor.
This chapter begins with some historical background to put NMR into perspective and concludes with a survey of the topics that we shall examine in the remainder of the book.
1.1 ORIGINS AND EARLY HISTORY OF NMR
Many atomic nuclei behave as though they are spinning, and as a result of this spin each nucleus possesses an angular momentum (p) and a magnetic moment (μ). These two nuclear properties were first observed indirectly in the very small splittings of certain spectral lines (hyperfine structure) in the visible and ultraviolet spectra of atoms. In 1924 Pauli2 suggested that this hyperfine structure resulted from the interaction of magnetic moments of nuclei with the already recognized magnetic moments of electrons in the atoms. Analysis of the hyperfine structure permitted the determination of the angular momentum and approximate magnetic moments of many nuclei. The concept of nuclear spin was strengthened by the discovery (through heat capacity measurements) of ortho and para hydrogen3—molecules that differ only in having the spins of the two constituent nuclei oriented in the same and opposite directions, respectively.
In the early 1920s Stern and Gerlach 4,5 had shown that a beam of atoms sent through an inhomogeneous magnetic field is split into two discrete beams because of magnetic effects arising from the quantized orbital angular momentum of the electrons. As the atoms move through the inhomogeneous field, the interaction between the magnetic moment of the atom and the magnetic field gradient causes the beam to deviate in either a positive or negative direction, depending on the quantum state of the electrons in the atom. During the 1930s, refinements of the Stern–Gerlach technique permitted the observation of much smaller effects from nuclear magnetic moments, and the laboratory of I. I. Rabi at Columbia University became a major center for such studies. With the development of improved techniques that employed three successive inhomogeneous magnets, as illustrated in Fig. 1.1, Rabi’s group was able to measure the signs and magnitudes of the magnetic moments of hydrogen and deuterium to an accuracy of about 5%.6
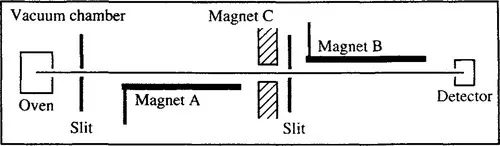
Figure 1.1 Schematic representation of the apparatus for molecular beam studies. Magnets A and B were electric wires that produce inhomogeneous magnetic fields. Magnet C was also an electric wire in initial experiments but was replaced by a magnet producing a homogeneous magnetic field for the resonance experiments. From Encyclopedia of Nuclear Magnetic Resonance, D. M. Grant and R. K. Harris, Eds. Copyright 1996 John Wiley & Sons Limited. Reproduced with permission.
In 1938 Rabi and his colleagues made a major improvement in beam experiments by substituting a very homogeneous magnet for the middle inhomogeneous magnet of Fig. 1.1 and by applying a radio frequency (rf) electromagnetic field to the molecules as they passed through the homogeneous field. With all molecules thus experiencing the same static magnetic field B, quantum theory showed that nuclear magnetic moments would interact with the field to give quantized energy levels separated by energy ∆E,
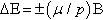
with the orientation of the spin differing in the two levels. Electromagnetic energy of a sharply defined frequency, υ = ∆E/h (where h is Planck’s constant), would then be absorbed by the nuclear spin system and cause a small but measurable deflection of the beam. The plot in Fig. 1.2 shows such a deflection and was the first observation of nuclear magnetic resonance.7 This work earned Rabi a Nobel Prize in 1944.
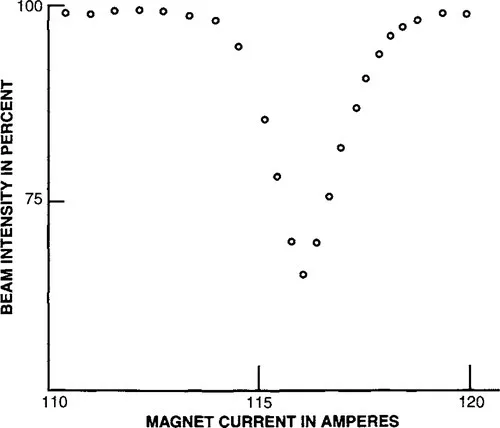
Figure 1.2 Plot of detector current resulting from refocused molecular beam as a function of the value of the field of magnet C. One ampere corresponds to 18.4 gauss. A radio frequency of 3.518 MHz was applied. From Rabi et al.7
With the resonance method, far higher precision in measurement of magnetic moments was possible, but such studies could be performed only in molecular beams under very high vacuum. The idea of observing NMR in bulk materials— solids, liquids, or even gases at normal pressure—was also considered. However, as we see in Chapter 2, the NMR signal in such circumstances is expected to be weak, and its observation would be difficult. In fact, in 1936 C. J. Gorter had attempted to detect resonance absorption by measuring the very slight increase in temperature of the sample on absorption of radio frequency energy. Although his experiment was unsuccessful (probably because of a poor choice of samples and experimental conditions), Gorter published the negative results8 and discussed them with Rabi. In fact, Gorter’s visit to Rabi’s laboratory in 1937 played a major role in Rabi’s decision to attempt to measure NMR in a molecular beam.9
It was not until 1945 that nuclear magnetic resonance was actually found in bulk materials. In that year Edward M. Purcell, Henry C. Torrey, and Robert V. Pound, three physicists who were completing wartime work on radar at the Massachusetts Institute of Technology (MIT), decided to look for resonance absorption from nuclear magnetic moments, using radio frequency techniques developed during World War II. Aware of Rabi’s work in molecular beams and of Gorter’s failed attempt in 1936 (and a second unsuccessful effort in 1942 using a different method), they nevertheless beli...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright page
- Preface to the Third Edition
- Chapter 1: Introduction
- Chapter 2: The Theory of NMR
- Chapter 3: Instrumentation and Techniques
- Chapter 4: Chemical Shifts
- Chapter 5: Coupling between Pairs of Spins
- Chapter 6: Structure and Analysis of Complex Spectra
- Chapter 7: Spectra of Solids
- Chapter 8: Relaxation
- Chapter 9: Pulse Sequences
- Chapter 10: Two-Dimensional NMR
- Chapter 11: Density Matrix and Product Operator Formalisms
- Chapter 12: Selected 1D, 2D, and 3D Experiments: A Further Look
- Chapter 13: Elucidation of Molecular Structure and Macromolecular Conformation
- Chapter 14: NMR Imaging and Spatially Localized Spectroscopy
- Appendix A: Properties of Common Nuclear Spins
- Appendix B: ABX and AA′XX′ Spectra
- Appendix C: Review of Relevant Mathematics
- Appendix D: Spin Matrices
- Appendix E: Selected Answers to Problems
- References
- Index