
eBook - ePub
Introduction to Biological and Small Molecule Drug Research and Development
Theory and Case Studies
- 472 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Introduction to Biological and Small Molecule Drug Research and Development
Theory and Case Studies
About this book
Introduction to Biological and Small Molecule Drug Research and Development provides, for the first time, an introduction to the science behind successful pharmaceutical research and development programs. The book explains basic principles, then compares and contrasts approaches to both biopharmaceuticals (proteins) and small molecule drugs, presenting an overview of the business and management issues of these approaches. The latter part of the book provides carefully selected real-life case studies illustrating how the theory presented in the first part of the book is actually put into practice. Studies include Herceptin/T-DM1, erythropoietin (Epogen/Eprex/NeoRecormon), anti-HIV protease inhibitor Darunavir, and more.
Introduction to Biological and Small Molecule Drug Research and Development is intended for late-stage undergraduates or postgraduates studying chemistry (at the biology interface), biochemistry, medicine, pharmacy, medicine, or allied subjects. The book is also useful in a wide variety of science degree courses, in post-graduate taught material (Masters and PhD), and as basic background reading for scientists in the pharmaceutical industry.
- For the first time, the fundamental scientific principles of biopharmaceuticals and small molecule chemotherapeutics are discussed side-by-side at a basic level
- Edited by three senior scientists with over 100 years of experience in drug research who have compiled the best scientific comparison of small molecule and biopharmaceuticals approaches to new drugs
- Illustrated with key examples of important drugs that exemplify the basic principles of pharmaceutical drug research and development
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Introduction to Biological and Small Molecule Drug Research and Development by C. Robin Ganellin,Roy Jefferis,Stanley M. Roberts in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Pharmacology. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
Introduction to enzymes, receptors and the action of small molecule drugs
Stanley M. Roberts∗ and Alasdair J. Gibb†, ∗School of Chemistry, Manchester University, Manchester M1 7ND, UK, †Research Department of Neuroscience, Physiology and Pharmacology, Division of Biosciences, University College London, London WC1E 6BT, UK
Abstract
Increasingly, pharmaceutical research and development is based on a detailed understanding of molecular interactions in diseased and healthy states of the human body. Over the past 50 years, most drug research has concentrated on the effects of small molecules on naturally occurring entities called enzymes and receptors. Hence, this chapter commences with an overview of the interactions of low-molecular-weight compounds (some natural [e.g. neurotransmitters] and some non-natural [e.g. drugs that inhibit certain enzymes]) with these natural macromolecules. This high-level introduction is followed by a more detailed inspection of the structures of some typical enzymes and receptors, emphasizing the complex shapes and subtle intermolecular interactions of these high-molecular-weight proteins. In addition, the importance of understanding the ‘on–off’ interaction between a small molecule and the target protein is illustrated by introducing the rate equations which dictate the kinetics of these episodes. The concluding section provides the first insight into the problems that have to be faced and overcome in moving from the point of having a compound with the desired effect on an enzyme or receptor in vitro to the position of introducing a useful drug to the marketplace.
Keywords/Abbreviations
Nerve cell; Neurotransmitter; Acetylcholine (receptor) (ACh(R)); Noradrenaline (NorA); Serotonin/5-hydroxytryptamine (5-HT); Dopamine (DA); Agonist/antagonist; Cyclic adenosine-3′,5′-monophosphate (cyclic-AMP); Cyclic guanosine -3′,5′-monophosphate (cyclic GMP); Adenosine/guanosine triphosphate (A/GTP); Catechol O-methyl transferase (COMT); Selective serotonin reuptake inhibitor (SSRI); Nicotinic/muscarinic AChR; Prodrug; Enzyme denaturation; Enzyme active/binding/catalytic sites; Co-enzyme; Enzyme cofactor; Michaelis–Menten equation; Lineweaver-Burk plot; L-DOPA; Allosteric enzyme inhibitor; Guanosine diphosphate (GDP); Hill-Langmuir equation; Scratchard plot; Lysergic acid diethylamide (LSD); G-protein coupled receptor (GPCR); Transmembrane (TM) domain; Gamma-aminobutyric acid (GABA); N-methyl-D-aspartate (NMDA); Sino-atrial (SA) node cell; Schild equation/plot; Efficacy
Chapter Outline
1.1 Section I: Background Information
1.1.1 Communication between cells: the roles of receptors and enzymes
1.1.2 Neurotransmitters, receptors and the nervous system
1.1.3 Introduction to enzymes and enzyme inhibitors
1.1.4 Other types of bioactive molecules
1.1.5 Factors influencing drug action
1.1.6 The impact of the sequencing of the human genome
1.2 Section II: More About Enzymes
1.2.1 Configuration of enzymes
1.2.2 Enzyme specificity, classification and nomenclature
1.2.3 Characteristics of enzyme catalysis
1.2.4 Enzyme reaction rates
1.2.5 Enzyme substrates as drugs
1.2.6 Enzyme inhibition and enzyme inhibitors as drugs
1.2.6.1 Irreversible inhibitors
1.2.6.2 Competitive inhibitors
1.2.6.3 Noncompetitive inhibitors
1.2.7 Enzyme regulation
1.3 Section III: More About Receptors
1.3.1 Bioassay and the measurement of drug effects
1.3.1.1 Bioassay
1.3.2 Quantifying drug–receptor interactions
1.3.3 Radioligand-binding studies – a direct measure of occupancy
1.3.4 Receptor structure
1.3.4.1 Nicotinic AChR structure: a ligand-gated ion channel
1.3.4.2 β-adrenoceptor structure: a G-protein-coupled receptor
1.3.5 Relating occupancy to response
1.3.4.1 Ligand-gated ion channels
1.3.4.2 Receptor mechanisms that involve second messengers
1.3.6 Competitive antagonism and the Schild equation
1.3.6.1 Drug blockade of open ion channels: a noncompetitive antagonism
1.3.7 Desensitization and the control of receptor number
1.3.8 Partial agonists, agonist efficacy and inverse agonism
1.4 Section IV
1.4.1 Conclusions: uncertainties in drug design and development
Further Reading
Acknowledgement
The section ‘More About Enzymes’ was adapted from Chapter 2 of the book ‘Medicinal Chemistry: the Role of Organic Chemistry in Drug Research’ (Academic Press, 1992) written by Dr Michael G. Davis. We acknowledge Dr Davis’s contribution.
1.1 Section I: Background Information
This chapter is adapted from the first three chapters of the book ‘Medicinal Chemistry: the Role of Organic Chemistry in Drug Research’ (eds. C. R. Ganellin and S. M.Roberts) Academic Press, London, 1992. While the basic principles remain the same, the text has been updated and modified to reflect the different focus of this book.
1.1.1 Communication between cells: the roles of receptors and enzymes
Some forms of life are composed of a single independent cell (the protozoa), while mammals are multicellular organisms. In between these two extremes there are life forms of varying complexity. All these organisms possess cell(s) to compartmentalize various chemical reactions in order to use available materials for energy and the maintenance of life’s processes.
Cells of different life forms have different characteristics (Figure 1.1) and, indeed, different cells from the same organism can be distinguished readily. For example, mammalian cells come in all shapes and sizes: compare the spheroidal leucocyte (the white blood cell), the flat epithelial cells found lining the mouth and the nerve cell (Figure 1.2).
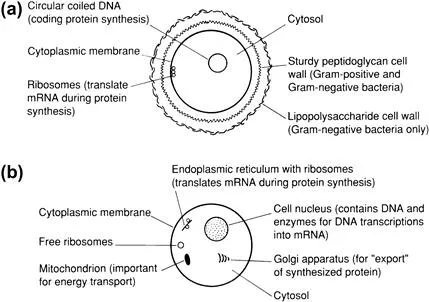
FIGURE 1.1 Simplistic representations of a prokaryotic bacterial cell (a) and a eukaryotic (possessing a nucleus) human cell (b) Not all substructures are shown.
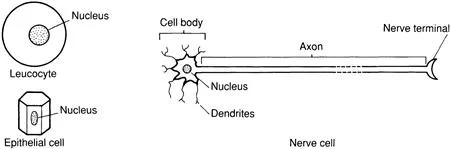
FIGURE 1.2 Shapes and sizes of mammalian cells.
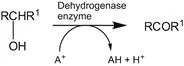
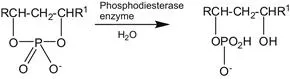
The cells are organized such that chemical transformations can be accomplished efficiently, the rate of these transformations being controlled by Nature’s catalysts – enzymes. Enzymes are high-molecular-weight compounds which catalyse anabolic (synthesis) and catabolic (degradation) reactions. The trivial name of the enzyme often gives a guide to its role (see Eqn 1.1–1.3); a more comprehensive list of enzyme activities is contained in Section 1.2.

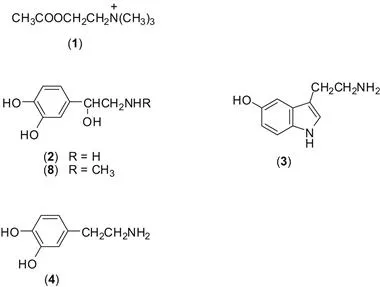
In order to coordinate their activities, the different cells in multicellular organisms need to communicate and this correspondence is accomplished mainly by small chemical molecules. For example, on receiving the appropriate signal, nerve terminals may release substances such as acetylcholine (ACh) (1), noradrenaline1 (NorA) (2), serotonin (3) (otherwise known as 5-hydroxytryptamine or 5-HT) or dopamine (DA) (4), and these substances, known as neurotransmitters, can interact with the appropriate receptors.
The receptors can lie, for example, on the surface of the cells opposite the nerve terminal (Figure 1.3). The interaction of a neurotransmitter (agonist2) with its receptor usually effects a change in conformation of the macromolecular receptor, leading to a change in enzyme activity within the cell (Figure 1.4), and/or movement of ions into or out of the cell (Figure 1.5).
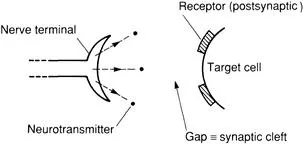
FIGURE 1.3 A neuroeffector junction (synapse).
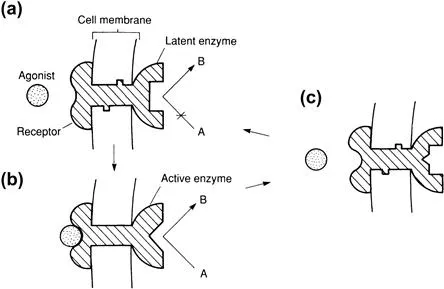
FIGURE 1.4 Activation of an enzyme by occupation of a receptor by an agonist. (a) Receptor free, enzyme inactive. (b) Receptor occupied, enzyme triggered into action (allosteric activation of enzyme). (c) Agonist leaves receptor surface and enzyme quickly returns to inactive form.
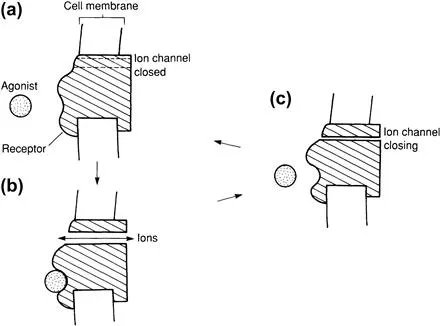
FIGURE 1.5 Opening ...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Biographies
- Preface
- Chapter 1. Introduction to enzymes, receptors and the action of small molecule drugs
- Chapter 2. Protein structure and function
- Chapter 3. The small molecule drug discovery process – from target selection to candidate selection
- Chapter 4. Protein therapeutics (introduction to biopharmaceuticals)
- Chapter 5. Similarities and differences in the discovery and use of biopharmaceuticals and small-molecule chemotherapeutics
- Chapter 6. Therapies for type 2 diabetes: modulating the incretin pathway using small molecule peptidase inhibitors or peptide mimetics
- Chapter 7. The structure and business of biopharmaceutical companies including the management of risks and resources
- Chapter 8. Discovery and development of the anticancer agent gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase
- Chapter 9. Targeting HER2 by monoclonal antibodies for cancer therapy
- Chapter 10. Recombinant human erythropoietin and its analogues
- Chapter 11. Lysosomal storage disorders: current treatments and future directions
- Chapter 12. Hormone replacement therapy
- Chapter 13. Design of the anti-HIV protease inhibitor darunavir
- Chapter 14. The case of anti-TNF agents
- Chapter 15. Discovery of the cholesterol absorption inhibitor, ezetimibe
- Colour Plate
- Index