
- 664 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Lithium-Ion Batteries features an in-depth description of different lithium-ion applications, including important features such as safety and reliability. This title acquaints readers with the numerous and often consumer-oriented applications of this widespread battery type.
Lithium-Ion Batteries also explores the concepts of nanostructured materials, as well as the importance of battery management systems. This handbook is an invaluable resource for electrochemical engineers and battery and fuel cell experts everywhere, from research institutions and universities to a worldwide array of professional industries.
- Contains all applications of consumer and industrial lithium-ion batteries, including reviews, in a single volume
- Features contributions from the world's leading industry and research experts
- Presents executive summaries of specific case studies
- Covers information on basic research and application approaches
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Lithium-Ion Batteries by Gianfranco Pistoia in PDF and/or ePUB format, as well as other popular books in Tecnologia e ingegneria & Energia. We have over one million books available in our catalogue for you to explore.
Information
1
Development of the Lithium-Ion Battery and Recent Technological Trends
Akira Yoshino, Yoshino Laboratory, Asahi Kasei Corp., 2-1, Samejima, Fuji-Shi, Shizuoka, Japan
Abstract
Lithium-ion batteries (LIBs) feature high energy density, high discharge power, and long service life. These characteristics facilitated a remarkable advance in portable electronics technology and the spread of information technology devices throughout society. Their emerging application to electric vehicles and large-scale storage systems make them a promising solution for challenges of environmental preservation and resource conservation. Research on LIBs started in the early 1980s, and the principle of the current LIB was completed in 1985. Since the LIB was first commercialized in 1991, battery performance has risen dramatically. Most of the technological developments to date have been directed toward the needs of portable electronics, but now the focus tends to be on the performance demands of medium- and large-scale applications. This chapter firstly describes the early technological innovations and then introduces and discusses the latest technology and research on the major battery components, the cathode, anode, electrolyte, and separator.
Keywords
Development; Lithium-ion battery; Origin; Technological trend
Chapter Outline
1. Introduction
2. Development of the Practical LIB
3. Development of Cathode Materials
3.1. History of Cathode Material Development
3.2. Recent Technological Trends of Cathode Materials
3.2.1. Three Morphologies of Cathode Materials
3.2.2. Layered Rock Salt Structure Materials (two dimensional)
3.2.3. Spinel Structure Materials (three dimensional)
3.2.4. Olivine Structure Materials (one dimensional)
3.3. Latest Research on Cathode Materials
3.3.1. Layered LCO Series (two dimensional)
3.3.2. Layered LiNiO2 Series (two dimensional)
3.3.3. Layered Mn Compound Series (two dimensional)
3.3.4. Spinel Structure Cathode Materials (three dimensional)
3.3.5. Olivine Structure Cathode Materials (one dimensional)
4. Development of Anode Materials
4.1. History of Anode Material Development
4.2. Recent Research on Anode Materials
5. Development of Electrolyte Solutions
5.1. History of Electrolyte Solution Development
5.2. Recent Research on Electrolyte Solutions
6. Separator Technology
6.1. Separator Production Methods and Characteristics
6.1.1. Dry-process One-component System
6.1.2. Wet-process Two-component System
6.1.3. Wet-process Three-component System
6.1.4. Shutdown Function
6.2. Recent Separator Developments
6.2.1. New Materials
6.2.2. Inorganic Coating
6.2.3. Separators Containing Inorganic Material
6.2.4. Nonwoven Separators
6.2.5. Laminated Separators
7. Conclusion
References
1 Introduction
In the 1980s, information technology (IT) advanced significantly with the development of portable electronic products such as video cameras, mobile phones, and notebook computers. This technological revolution led to a growing need for rechargeable batteries with greater capacity or with reduced size and weight for a given capacity. Conventional rechargeable batteries available or under development at that time such as lead–acid, nickel–cadmium, and nickel–metal hydride batteries used aqueous electrolytes, which posed limitations on increasing the energy density and reducing the size and weight. Thus, there remained an unmet need for a new, small and lightweight rechargeable battery to be put into practical use. Research on the lithium-ion battery (LIB) started in the early 1980s, and the first commercialization was achieved in 1991. Since then, LIBs have grown to become the dominant power storage solution for portable IT devices. The LIB market has continued to expand rapidly for over 15 years, and its worldwide scale now exceeds JP Yen 106 millions ($13,000 millions) as shown in Figure 1.1.
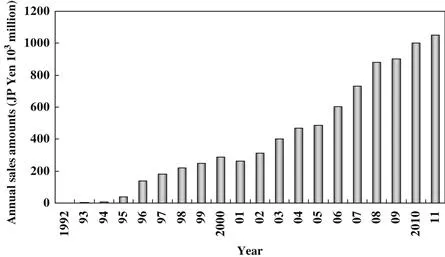
FIGURE 1.1 Expansion of worldwide LIB demand.
The four major components of the LIB are the cathode, anode, electrolyte, and separator. LIBs generally produce an average cell voltage of around 3.7 V and operate on the relatively simple principle of reversible intercalation of Li ions in the cathode and anode. The most commonly used material for the cathode is lithium cobalt oxide, LiCoO2, and some form of carbon is generally used for the anode. In the completely discharged state, Li atoms are only contained as part of the cathode. On charging, Li ions are released from the cathode and migrate through the electrolyte into the carbon of the anode. The reverse reaction occurs during discharging, and electric energy is stored or released by repeating these reactions reversibly. The rapidly growing LIB market described above owes to this safe and efficient principle. This chapter focuses on two subjects: the development of the first practical LIB configuration in the 1980s and the recent technological trends for LIBs. The principle of the present commercial LIB as described above was completed in 1985 with a patent filed by the author [1]. This invention and other successive patents illustrate the origins of the key LIB components and configuration, as well as the technological requirements associated with them. Since many of the requirements at the time are analogous to the objectives of current research, reviewing the initial stage of LIB development will provide rich context for and insight into the overall technology. Following a brief overview of the initial development, sections devoted to each major LIB component will begin with a description of the early technological innovations followed by a discussion of recent technology and research.
2 Development of the Practical LIB
The author and colleagues focused on creating a practical new nonaqueous electrolyte rechargeable battery to meet the emerging need for a small and lightweight power source for portable electronics. Our essential achievements made in the 1980s were as follows: (1) proposition of fundamental technology for composition of the LIB, in which LiCoO2 is used as the cathode and a carbonaceous material with a certain crystalline structure is used as the anode; (2) invention of essential constituent technologies for the electrodes, electrolyte, and separator; and (3) development of peripheral technology such as safety device technology, protective circuit technology, and charging and discharging technology.
The first step to develop the practical LIBs was the adoption of LiCoO2 for the cathode. LiCoO2 was first disclosed by Goodenough et al. [2,3] and it remains the most commonly used cathode material at present. One anode material that was gaining attention at the time was graphite [4], but it was known that propylene carbonate, which was then the common organic electrolyte solvent, would decompose during charging when graphite was used. Furthermore, the use of a solid electrolyte resulted in electrical resistance which was too high to enable practical charging and discharging. We created a working model LIB using LiCoO2 as the cathode and polyacetylene as the anode, but rejected polyacetylene due to its low density (high bulk) which precluded reduction of cell size. Studying several carbonaceous materials for their suitability as anode, we found that a carbonaceous material with a certain crystalline structure provided greater capacity without causing decomposition of the propylene carbonate electrolyte solvent, as graphite did. The secondary battery which we successfully fabricated based on this new combination of component materials enabled stable charging and discharging, over many cycles for a long period [1].
This combination of electrode materials marked a new concept of a secondary battery based on the transfer of Li ions. Cell reaction without chemical transformation provided stable battery characteristics over a long service life, including excellent cycle durability with little degradation by side reactions, and excellent storage characteristics. Furthermore, this development also enabled simple and efficient assembly in the discharged state, with no special atmosphere required because LiCoO2 is very stable in air, despite containing Li ions, and the anode is composed of carbonaceous material which is also stable.
Another key step was the development of essential constituent technologies including technology for fabricating electrodes and technology for assembling batteries. In the basic structure of the typical LIB, a multilayer electrode assembly (electrode coil), prepared by winding sheets of cathode and anode with separator membrane in between, is inserted into a battery can. This is then infused with nonaqueous electrolyte solution composed of LiPF6 or LiBF4 dissolved in a mixture of carbonate compounds and sealed. Both the cath...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Contributors
- Preface
- 1. Development of the Lithium-Ion Battery and Recent Technological Trends
- 2. Past, Present and Future of Lithium-Ion Batteries: Can New Technologies Open up New Horizons?
- 3. Fast Charging (up to 6C) of Lithium-Ion Cells and Modules: Electrical and Thermal Response and Life Cycle Tests
- 4. Nanostructured Electrode Materials for Lithium-Ion Batteries
- 5. EVs and HEVs: The Need and Potential Functions of Batteries for Future Systems
- 6. Manufacturing Costs of Batteries for Electric Vehicles
- 7. Lithium-Ion Battery Packs for EVs
- 8. The Voltec System—Energy Storage and Electric Propulsion
- 9. Transit Bus Applications of Lithium-Ion Batteries: Progress and Prospects
- 10. EVs and HEVs Using Lithium-Ion Batteries
- 11. The Challenge of PHEV Battery Design and the Opportunities of Electrothermal Modeling
- 12. Solid-State Lithium-Ion Batteries for Electric Vehicles
- 13. Lithium-Ion Batteries for Storage of Renewable Energies and Electric Grid Backup
- 14. Satellite Lithium-Ion Batteries
- 15. Lithium-Ion Battery Management
- 16. Electronic Options for Lithium-Ion Batteries
- 17. Safety of Commercial Lithium-Ion Cells and Batteries
- 18. Safety of Lithium-Ion Batteries
- 19. Lithium-Ion Cell Components and Their Effect on High-Power Battery Safety
- 20. Thermal Stability of Materials in Lithium-Ion Cells
- 21. Lithium-Ion Battery Environmental Impacts
- 22. Recycling of Traction Batteries as a Challenge and Chance for Future Lithium Availability
- 23. Manufacturers, Materials and Recycling Technologies
- 24. The Lithium-Ion Battery Value Chain—Status, Trends and Implications
- 25. Thermodynamics of Lithium-Ion Batteries
- Color Plate Section
- Index