
- 736 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Most of the diseases of modern mankind involve either acute or chronic inflammation. Measuring Immunity integrates the current information available on biomarkers and surrogate assays into a single handbook. It highlights the principles behind various applications, gives a brief summary on how they are conducted and provides detailed and critical analyses of murine models of immunity, clinical trials, and tests to predict utility and benefit. Measuring Immunity is indispensable for scientists and clinicians interested in the clinical applications of modern immunobiology.* Defines which assays of immune function are helpful in the assessment of clinical disorders involving inflammation and immunity* Assesses the dynamics of cellular and soluble factors in the peripheral blood using modern techniques * Includes basic science foundations as well as the approaches currently applied
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Topic
MedicineSubtopic
ImmunologySection VI
Assays in Acute and Chronic Diseases
Chapter 40
Cancer – Solid Tumors
Mary L. Disis, the Immunologic Monitoring Consortium Tumor Vaccine Group, University of Washington, Seattle, WA, USA
The great tragedy of science – the slaying of a beautiful hypothesis by an ugly fact.
Thomas Huxley
INTRODUCTION
Recent advances in molecular biology and basic immunology have resulted in the development of highly quantitative assays to measure tumor-specific T cell immunity. The definition of specific tumor antigens and a more detailed understanding of T cell-antigen recognition and the character of peptides presented in the major histocompatibitiy complex (MHC) have resulted in the development of novel methods to quantitate the human T cell response to cancer. As compared to older methods of immune response analysis such as delayed type hypersensitivity (DTH) response, chromium release assays (51Cr) and tritiated thymidine incorporation, new immunologic monitoring techniques are, in general, more robust and reproducible (Table 40.1).
Table 40.1
Comparison of assay characteristics
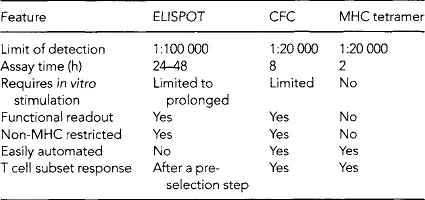
Currently there are no standard methods of assessing immunity after an immunotherapeutic intervention. The ideal immunologic assay would be one whose detection of immunity correlated with a clinical response and could be adapted to wide scale clinical use. The amount of clinical material that can be obtained on individual cancer patients would limit the choice of assay used (Table 40.2). Currently, clinical trials of immunotherapeutics should be designed to incorporate validation of any particular biomarker into the statistical assessment correlating laboratory results with clinical outcome. As we wait and see whether any particular assay will be predictive of clinical outcome, many immunologic monitoring methods have proven to be of utility as a measure of potency. Vaccines designed to elicit a specific immune response, e.g. to class I peptides or a Th1 response, can be analyzed exquisitely to determine whether immunization resulted in the desired outcome. In addition, reproducible immunologic monitoring methods have allowed comparison of different vaccine strategies as well as the identification and characterization of baseline immunity in cancer patients.
Table 40.2
Cellular requirements for common assays per a single antigen and control
| Assay type | Amount of PBMC needed |
| LDA | 50 × 106 |
| ELISPOT | 10 × 106 |
| Flow based assays (CFC, tetramer) | 1 × 106 |
Evaluation of cytokine production or expression after antigenic stimulation via ELISPOT or cytokine flow cytometry (CFC) and assessment of a clonal T cell response in terms of measuring T cell binding to particular MHC-peptide complexes have become standard immunologic tools for measuring human tumor-specific T cell immunity. The studies described below are representative examples of the types of analysis that can be performed evaluating and characterizing tumor specific immunity in patients with solid tumors.
ASSESSMENT OF CELLULAR IMMUNITY BASED ON ANTIGEN SPECIFIC CYTOKINE PRODUCTION
Antigen specific immune responses are largely regulated by secretion of cytokines whose function is to govern the growth and differentiation of T cell populations. Secretion of cytokines by T cells responding to a specific antigen has become a common measure of a functional immune response. Although the measurement of cytokine production by T cells using ELISA methodologies is not quantitative, measurement of cytokines offers a detailed characterization of the function of the T cell and the phenotype of the immune environment generated after antigen recognition. Evaluation of cytokine production by an individual T cell can be assessed using either ELISPOT or CFC. Thus, these assays are a highly quantitative measure of potentially functional T cells.
ELISPOT
Cytokine release assays have been modified to provide a quantitative measure of precursor frequency. Recently, enzymelinked immunosorbent-spot (ELISPOT) has been used to characterize cytokine release at the single cell level (Scheibenbogen et al., 1997, 2003; Schmittel et al., 1997; Vaquerano et al., 1998). ELISPOT is a sensitive T cell quantitation method and can detect specific precursors at 1:100 000. Indeed, ELISPOT is being increasingly used as a tool for monitoring immunologic responses to cancer vaccines (Wang and Rosenberg, 1999; Asai et al., 2000; Meidenbauer et al., 2000). The methods individual laboratories employ for ELISPOT vary greatly (Asai et al., 2000). Standardization of technique and definition of the sensitivity and range of the assay are needed to develop ELISPOT as a clinical tool.
ELISPOT analysis has been used by several investigators to define and characterize baseline T cell immunity to specific tumor antigens in a variety of solid tumor patient populations. Tatsumi et al. (2002) used interferon (IFN)-gamma and interleukin (IL)-5 ELISPOT assays on fractionated T cell populations to examine the magnitude of Th1 and Th2 responses to HLA-DRB1*0401 epitopes of MAGE-6. They demonstrated that patients with active melanoma or renal cell carcinoma displayed a strongly polarized Th2-type reactivity to these peptides, while normal donors and patients who were disease-free following therapeutic intervention exhibited either a weak mixed Th1/Th2-type or strongly polarized Th1-type response to the same epitopes. It should be emphasized that these polarized CD4+ responses are specific for these specific epitopes and do not represent a general tendency of the donor or patient to respond in a generically Th2-biased or Th1-biased fashion. Mitogen treatment of peripheral blood mononuclear cells (PBMC) stimulated IFN-gamma and IL-5 responses that were indistinguishable between the patients with active melanoma or renal cell carcinoma and normal donors and patients who were disease-free. While the numbers of patients in the study were relatively small, the results do imply that a Th2-type dominated CD4+ response against the MAGE-6 epitopes may correlate with active disease status. Investigators are extending these studies to determine the prognostic significance of Th1-type and Th2-type balance. Studies of baseline immunity to tumor antigens may also indicate differences in immune responses to the same antigens between tumor types. Investigators evaluated T cell responses against Ep-CAM, HER-2/neu and CEA in patients with breast and colorectal cancer by IFN-gamma ELISPOT. Although influenza specific responses were similar in both populations, tumor antigen specific T cell immunity could only be detected in the colorectal cancer patients (Nagorsen et al., 2003).
ELISPOT has also been used to assess the potency of a particular vaccine approach. Vaccine studies comparing vaccination strategies and adjuvants have utilized ELISPOT to determine which approach to be superior. One study evaluated DNA plasmid, peptide and modified vaccinia Ankara virus (MVA) combinations in their ability to elicit influenza specific responses in mice (Woodberry et al., 2003). Investigators demonstrated while DNA/MVA combinations could enhance IFN-gamma secretion by CD8+ T cells, protection against infection was not enhanced. Studies such as this, in preclinical models, will influence the design of human vaccine trials. Vaccine comparative evaluations are already ongoing in humans. For example, investigators vaccinated melanoma patients with tyrosinase peptides in granulocyte macrophage colony stimulating factor (GM-CSF), keyhole limpit hemocyanin (KLH), or both (Scheibenbogen et al., 2003). Using IFN-gamma ELISPOT as a biomarker, they determined that the use of both adjuvants resulted in the generation of immunity in a larger number of patients than either adjuvant alone.
Finally, human clinical trials of cancer vaccines suggest that a positive ELISPOT response may correlate with improved clinical outcome after vaccination. Evidence of a correlation between immunologic responses defined by the ELISPOT assay and clinical response has been reported in a recent study in melanoma patients testing tumor derived heat shock protein gp96-peptide complexes (Belli et al., 2002). Notably, this group measured responses using freshly isolated PBMC without any in vitro culture step and used a stringent definition of a positive ELISPOT (Keilholz et al., 2002). The study showed a strong correlation (P< 0.01) between T cell responses meas...
Table of contents
- Cover image
- Title page
- Table of Contents
- Dedication
- Copyright
- About the Authors
- Foreword: THE BEDSIDE IS THE BENCH
- Preface
- Contributors
- Section I: Fundamentals of the Immune Response
- Section II: Serologic Assays
- Section III: Cellular Enumeration and Phenotyping
- Section IV: Cellular Function and Physiology
- Section V: Provocative Assays in vivo
- Section VI: Assays in Acute and Chronic Diseases
- Section VII: New Technologies
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Measuring Immunity by Michael T. Lotze,Angus W. Thomson in PDF and/or ePUB format, as well as other popular books in Medicine & Immunology. We have over one million books available in our catalogue for you to explore.