
- 1,218 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Photochromism: Molecules and Systems
About this book
Photochromism is simply defined as the light induced reversible change of colour. The field has developed rapidly during the past decade as a result of attempts to improve the established materials and to discover new devices for applications.As photochromism bridges molecular, supramolecular and solid state chemistry, as well as organic, inorganic and physical chemistry, such a treatment requires a multidisciplinary approach and a broad presentation. The first edition (1990) provided an enormous amount of new concepts and data, such as the presentation of main families based on the pericyclic reaction mechanism, the review of new families, some bimolecular photocycloadditions and some promising systems. This new edition provides an efficient entry into this flourishing field, with the core content retained from the original work to provide a basic introduction into the different subjects.
- Second edition of a work first published in 1990, now revised due to constant development of research. *Including updated lists of references (1989-2001), offering immediate access to recent developments
- Providing great basic interest and high application potential bringing scientists together from chemistry, physics and engineering
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Photochromism: Molecules and Systems by Heinz Dürr,Henri Bouas-Laurent in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Organic Chemistry. We have over one million books available in our catalogue for you to explore.
Information
Chapter 5
4n Systems Based on 1,3-Electrocyclization
C. Schulz
H. Dürr
1 INTRODUCTION
The photochromic behavior of many classes of compounds is based on electrocyclizations. In this chapter, photochromic systems with four electrons which undergo 1,3-electrocyclic reactions are dealt with. 1,5-Electrocyclizations involving six electrons are described elsewhere.
In scheme 1, a survey of electrocyclic processes in 4n-systems (n = 1,2,…) is shown.
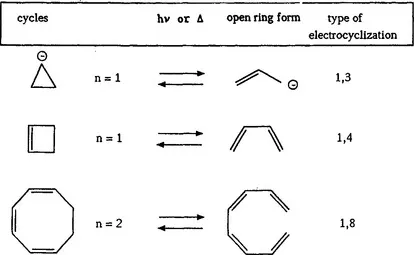
Scheme 1 Electrocyclic Processes in 4n-Systems (n = 1,2,…)
Important photochromic heterocycles and open ring forms isoelectronic with the cyclopropyl and allyl anion, respectively, are given in scheme 2.
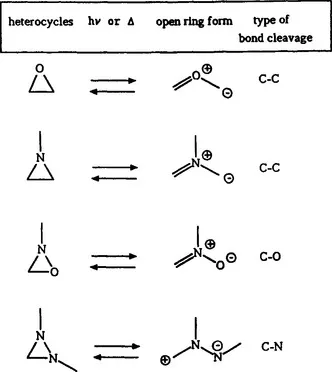
Scheme 2 Photochromism based on 1,3-electrocyclization (4 electrons)
The cyclobutene-butadiene interconversion, where a wealth of derivatives has been studied both theoretically and experimentally, has not given rise to important photochromic systems. This reaction is therefore not treated in this book. The reader is referred to earlier literature (ref. 1). Similar arguments hold for the cyclooctatriene-octatetraene interconversion which is excluded from this book as well.
2 STEREOCHEMISTRY IN 1,3-ELECTROCYCLIC REACTIONS
An outstanding feature of electrocyclic reactions is their stereospecificity. The orbital symmetry conservation principle in the form of the Woodward-Hoffmann rules (ref. 2) predicts the following behavior for symmetrical hydrocarbons: When the number of interacting electrons in the cyclic array is 4n (n = 1,2,.) the thermal electrocyclic reactions proceed via conrotatory pathways, the electrocyclic reactions which occur as primary photochemical processes via disrotatory pathways.
The rules may be applied to slightly disturbed systems. The question which arises concerns the application of the Woodward-Hoffmann rules to unsymmetrical substances including both highly substituted hydrocarbons as well as compounds containing heteroatoms. A paper by Snyder (ref. 3) describes the CNDO-calculated electrocyclization of the open ring form 1, isoelectronic with the allyl anion, to the corresponding three membered ring 2.
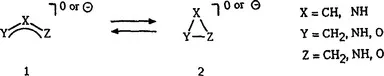
The results of these calculations show that heteroatom substitution of 1, 2. leads to three types of ring closures:
1. Thermal conrotation is allowed:
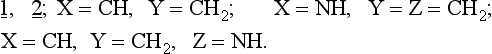
2. Thermal conrotation and disrotation are allowed:
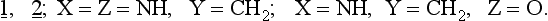
3. Conrotation and disrotation are thermally forbidden but photochemically allowed:
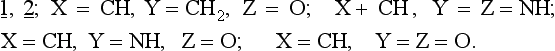
3 OXIRANES
3.1 Monocyclic Aryloxiranes
The literature on oxiranes has been reviewed (refs. 4–6). It has been shown that room temperature photolysis of aryloxiranes (incorporating 3) causes cycloelimination to give arylcarbenes and carbonyl compounds.
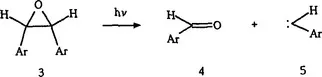
Photolysis (77 K) in rigid glasses produces highly colored intermediates (ref. 7), which are stable at low temperatures, but are bleached by warming to 25 °C or upon irradiation with visible light. Color formation is attributed to C-C-bond cleavage with formation of carbonyl ylides 7.
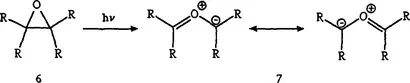
Thap DoMinh, Trozzolo and Griffin (ref. 8) investigated the photochromism of cis- and trans-stilbene oxide. In the present review these compounds are treated as typical examples of other photochromic monocyclic oxiranes.
Irradiation of trans-stilbene oxide in ethanol glass at 77 K produces an orange material (λmax = 490 nm) along with small amounts of benzaldehyde, phenylmethylene and desoxybenzoin. Irradiation of the cis isomer gives similar products, but the colored intermediate is a deep red compound (λmax = 510 nm). At 140 K, both colors disappear (the bleaching occurring somewhat faster in the trans than in the cis isomer) giving benzaldehyde and phenylmethylene. The amount of fragmentation products formed by this photolysis-warm-up-procedure is estimated to be 20–25 times more than the amount originally produced by photolysis. Irradiation in the visible causes rapid fading and regenerates the original oxirane together with small amounts of fragmentation products.
The process may be envisaged as an electrocyclic reaction which interconverts oxiranes and open chain carbonyl ylides by a conrotatory or disrotatory mode. Three possible ylides may be derived from stilbene oxides: The order of stability is 9 > 8 >> 10. From the relative stabilities and absorption spectra it is possible to assign cis-oxoylide 9 to the more stable red shifted intermediate from cis-stilbene o...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Preface (1990 Edition)
- Foreword (2003 Revised Edition)
- Preface (2003 Revised Edition)
- See Literature Survey Update (1989 – 2001)
- ORGANIC PHOTOCHROMISM (IUPAC Technical Report)
- General Introduction
- Photophysical, Photochemical and Photokinetic Properties of Photochromic Systems
- Photochromism Based on “E-Z” Isomerhation of Double Bonds
- Photochromism Based on Pericyclic Reactions: Electrocyclization Reactions
- Photochromism Based on Pericyclic Reactions: Cycloaddition Reactions
- Photochromism Based on Tautomerism (Hydrogen Transfer)
- Photochromism Based of Dissociation Processes
- Photochromism in Biological Systems
- Environmental Effects on Organic Photochromic Systems
- The Use of Silver Salts for Photochromic Glasses
- Applications
- New Developments Highly Promising for Applications
- Glossary of Terms
- Appendix of Literature Updates
- Subject Index