
- 448 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Metal Oxides in Energy Technologies
About this book
Metal Oxides in Energy Technologies provides, for the first time, a look at the wide range of energy applications of metal oxides. Topics covered include metal oxides materials and their applications in batteries, supercapacitors, fuel cells, solar cells, supercapacitors, and much more. The book is written by an experienced author of over 240 papers in peer-reviewed journals who was also been recognized as one of Thomson Reuter's "World's Most Influential Scientific Minds in 2015. This book presents a unique work that is ideal for academic researchers and engineers.- Presents an authoritative overview on metal oxides in energy technologies as written by an expert author who has published extensively in the area- Offers up-to-date coverage of a large, rapidly growing and complex literature- Focuses on applications, making it an ideal resource for those who want to apply this knowledge in industry
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Introduction: Energy technologies and their role in our life
† Department of Chemistry, University of Venda, Thohoyandou, South Africa
Abstract
Keywords
Acknowledgment
1.1 Preparation methods of metal oxides and energy technologies
- • High-temperature solid-state (ceramic) method. A simple method in which the solid precursors are mixed intimately and then reacted at a high temperature [1].
- • Hydrothermal method. A solution of the precursor is heated in an autoclave to produce a crystalline product [2, 3].
- • Templating method. Organic materials especially polymers such as polystyrene are used to form a template with a distinct morphology and then a metal oxide is deposited into the empty space to form a porous material after the removal of the template [4, 5].
- • Sol-gel method. A sol is formed by the precursors, usually by means of hydrolysis and condensation reactions. After the sol polymerizes to form a gel, the gel is dried and heated to form the nanomaterial. In some cases, carriers such as organic complex can be added [6, 7].
- • Electrodeposition. A suspension of metal ions and insoluble particles is deposited electrochemically on an inert cathode surface [8, 9].
- • Chemical vapor deposition. The low-deposition temperature combined with a high-deposition rate produces a solid membrane with controllable composition and crystallinity [10, 11].
- • Chemical precipitation. An insoluble metal oxide is formed by chemical reaction between different solutions [12, 13].
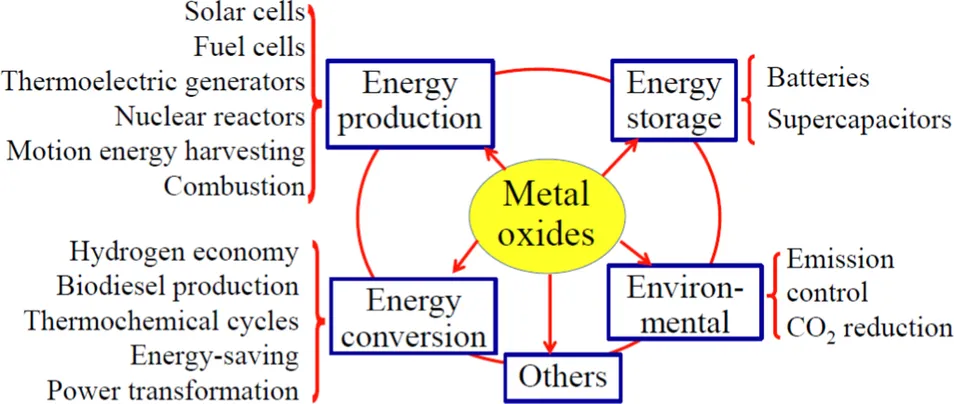
1.2 Energy generation
1.2.1 Solar cells
1.2.2 Fuel cells
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- List of contributors
- About the Series Editor
- About the author
- Preface to the series
- Preface
- 1: Introduction: Energy technologies and their role in our life
- 2: Metal oxides in fuel cells
- 3: Metal oxide-based thermoelectric materials
- 4: Mixed oxides in nuclear fuels
- 5: Piezoelectric energy harvesting systems with metal oxides
- 6: Metal oxides in batteries
- 7: Metal oxides in supercapacitors
- 8: Metal oxide semiconductors for solar water splitting
- 9: Metal oxides for hydrogen storage
- 10: Requirements for efficient metal oxide photocatalysts for CO2 reduction
- 11: Metal oxide catalysts for biodiesel production
- 12: Solar-driven fuel production by metal-oxide thermochemical cycles
- 13: Metal oxides in energy-saving smart windows
- 14: Metal oxide-based superconductors in AC power transportation and transformation
- 15: Metal oxides for emission control
- Index