
eBook - ePub
Hybrid Microcircuit Technology Handbook
Materials, Processes, Design, Testing and Production
- 600 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Hybrid Microcircuit Technology Handbook
Materials, Processes, Design, Testing and Production
About this book
The Hybrid Microcircuit Technology Handbook integrates the many diverse technologies used in the design, fabrication, assembly, and testing of hybrid segments crucial to the success of producing reliable circuits in high yields. Among these are: resistor trimming, wire bonding, die attachment, cleaning, hermetic sealing, and moisture analysis. In addition to thin films, thick films, and assembly processes, important chapters on substrate selections, handling (including electrostatic discharge), failure analysis, and documentation are included. A comprehensive chapter of design guidelines will be of value to materials and process engineers, chemists, and electrical engineers who design and test hybrid circuits.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Hybrid Microcircuit Technology Handbook by James J. Licari,Leonard R Enlow in PDF and/or ePUB format, as well as other popular books in Technik & Maschinenbau & Elektrotechnik & Telekommunikation. We have over one million books available in our catalogue for you to explore.
Information
1
Introduction
1.0 CLASSIFICATION OF MATERIALS FOR MICROELECTRONICS
Before proceeding to a discussion of hybrid circuit and multichip module processes, it is important to have a general understanding of the materials most commonly used in today’s electronic equipment. Indeed, it is difficult to dissociate processes from materials. All electronic devices and circuits are based on combinations of three general types of materials. These are conductors, insulators, and semiconductors, and are defined in terms of their abilities to either conduct or impede the flow of electrons.
1.1 Conductors
Conductivity is a function of the number of free electrons and their mobility. Conductors conduct electricity readily because they have many free and mobile electrons available to carry the current. The best electrical conductors are metals, generally elements residing in Groups I, II, and III of the Periodic Table. Elements in Group IB, typically metals having a valence of 1, are most conductive. Because these elements contain only one electron in their outermost shells, the electron is easily released from the forces of attraction of the nucleus. Thus, elements of Group IB (copper, silver, and gold) are among the best conductors known and the most widely used in electronics. Metals with valences of 2 or 3 are also good conductors, but not as good as those of Group IB. Among these are aluminum, palladium, platinum, nickel, and tungsten. A comparison of the resistivities (inverse of conductivities) of various metals is given in Table 1. The metals and alloys most widely used in the fabrication of electronic circuits and systems and their general applications are given in Table 2.
Table 1
Electrical Resistivities of Metal Conductors.
| Metal | Symbol | Resistivity (ohm-cm × 10−6) |
| Silver | Ag | 1.62 |
| Copper | Cu | 1.69 |
| Gold | Au | 2.38 |
| Aluminum | Al | 2.62 |
| Tungsten | W | 5.52 |
| Nickel | Ni | 6.9 |
| Indium | In | 9.0 |
| Platinum | Pt | 10.52 |
| Palladium | Pd | 10.75 |
| Tin | Sn | 11.5 |
| Tin-lead | Sn-Pb (60–40) | 14.99 |
| Lead | Pb | 20.65 |
Table 2
Commonly Used Conductors and Their Uses in Electronics.
| Conductor | Symbol | Use |
| Gold | Au | Wire, plating, thick films, thin films, filler in epoxy conductive adhesives for device attachment |
| Gold-platinum | Au-Pt | Thick-film solderable conductors |
| Gold-palladium | Au-Pd | Thick-film solderable conductors |
| Gold-silver | Au-Ag | Low-cost thick-film solderable conductors |
| Gold-silicon | Au-Si | Eutectic attachment of devices for ohmic contact |
| Gold-germanium | Au-Ge | Eutectic attachment of devices for ohmic contact |
| Silver | Ag | Low-cost conductor, thick-film plating, thin film, EMI, filler for epoxy conductive adhesives |
| Silver-palladium | Ag-Pd | Thick film (low silver migration) |
| Silver-platinum | Ag-Pt | Thick film (low silver migration) |
| Copper | Cu | Thick film (low cost), thin film, foil and plating for printed circuit boards, wire, filler for epoxy conductive adhesives |
| Aluminum | Al | Wire, thin-film metallization for devices |
| Tungsten | W | Thick-film conductors for co-fired tape substrates and packages |
| Molybdenum-manganese | Mo-Mn | Thick-film conductors for co-fired tape substrates and packages |
| Nickel | Ni | Barrier metal, low conductivity metal, plating |
| Tin-lead alloys | Sn-Pb | Solder for attachment of components, plating for circuit boards |
| Indium and alloys | In | Low-temperature solder for attachment of devices and components |
| Tin-silver alloys | Sn-Ag | Low-temperature solders |
Metals have a positive temperature-coefficient-of-resistivity (TCR); that is, as the temperature of a conductor increases, its resistivity increases or, conversely, its conductivity decreases. However, for the highly conductive metals, (Al, Au, Ag), the effect is not significant in the temperature range over which circuits are tested or used (room temperature to about 150°C), Fig. 1.
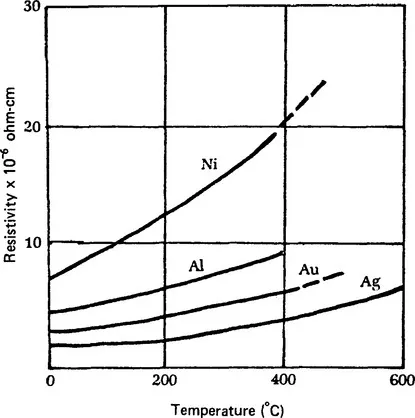
Figure 1 Electrical resistivity of metals versus temperature. (note positive TCRs)
1.2 Insulators
At the other end of the spectrum from conductors are the insulators, which are very poor conductors of electricity. Their valence electrons are tightly bound and not free to move within the structure. In an insulator, the bonds that hold the atoms together, as a rule, are covalent (shared electrons) and possess high bond strengths (the energy in kilocalories per mole to break apart a bond pair). An important class of insulators are compounds based on the carbon atom—hydrocarbons, polymers, and plastics fall within this group. The bond energies for C-C, C-H, and C-O bonds are 83, 99, and 110.2 kcal/mol, respectively—thus extremely difficult to break apart to create free-moving electrons. Sufficient energy, of course, could be applied in the form of extreme heat or radiation to eventually rupture the bonds, but this is done only at the expense of causing irreversible damage to the material. Free radicals and smaller molecular fractions would then be formed which are quite different in physical properties from the original material.
Insulators generally reside in Groups V, VI, and VII, of the Periodic Table. Examples of excellent insulators include: plastics, rubber, ceramics, glass, sapphire (a form of aluminum oxide), other metal oxides (aluminum oxide, beryllium oxide, and silicon dioxide), and metal nitrides (silicon nitride, aluminum nitride). Insulators may be solids, liquids, or gases, but only solids are extensively used in electronics. Applications include substrates on which resistors, capacitors, and metal conductors are formed, substrates within which semiconductive, resistive, and conductive areas are generated (ICs), and passivation layers protecting active circuits from moisture and other harsh environments. Solid insulators have resistivities ranging from several megohm-cm to greater than 1014 ohm-cm, but caution should be used in their selection because in some cases the insulating properties can be rapidly degraded by ionic impurities, moisture absorption, or thermal and radiation exposures. In contradistinction to metals, insulators have negative TCRs (resistivity decreases with increase in temperature). For the ceramics that are most widely used as circuit substrates or as dielectric insulating layers, this decrease is signifi...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- MATERIALS SCIENCE AND PROCESS TECHNOLOGY SERIES
- Preface to the Second Edition
- Preface to the First Edition
- NOTICE
- Chapter 1: Introduction
- Chapter 2: Substrates
- Chapter 3: Thin Film Processes
- Chapter 4: Thick Film Processes
- Chapter 5: Resistor Trimming
- Chapter 6: Parts Selection
- Chapter 7: Assembly Processes
- Chapter 8: Testing
- Chapter 9: Handling and Clean Rooms
- Chapter 10: Design Guidelines
- Chapter 11: Documentation and Specifications
- Chapter 12: Failure Analysis
- Chapter 13: Multichip Modules: A New Breed of Hybrid Microcircuits
- Index