A Combined MRI and Histology Atlas of the Rhesus Monkey Brain in Stereotaxic Coordinates
- 402 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
A Combined MRI and Histology Atlas of the Rhesus Monkey Brain in Stereotaxic Coordinates
About this book
A Combined MRI and Histology Atlas of the Rhesus Monkey Brain in Stereotaxic Coordinates, Second Editionmaps the detailed architectonic subdivisions of the cortical and subcortical areas in the macaque monkey brain using high-resolution magnetic resonance (MR) images and the corresponding histology sections in the same animal. This edition of the atlas is unlike anything else available as it includes the detailed cyto- and chemoarchitectonic delineations of the brain areas in all three planes of sections (horizontal, coronal, and sagittal) that are derived from the same animal. This is a significant progress because in functional imaging studies, such as fMRI, both the horizontal and sagittal planes of sections are often the preferred planes given that multiple functionally active regions can be visualized simultaneously in a single horizontal or sagittal section. This combined MRI and histology atlas is designed to provide an easy-to-use reference for anatomical and physiological studies in macaque monkeys, and in functional-imaging studies in human and non-human primates using fMRI and PET.- The first rhesus monkey brain atlas with horizontal, coronal, and sagittal planes of sections, derived from the same animal- Shows the first detailed delineations of the cortical and subcortical areas in horizontal, coronal, and sagittal plane of sections in the same animal using different staining methods- Horizonal series illustrates the dorsoventral extent of the left hemisphere in 47 horizontal MRI and photomicrographic sections matched with 47 detailed diagrams (Chapter 3)- Coronal series presents the full rostrocaudal extent of the right hemisphere in 76 coronal MRI and photomicrographic sections, with 76 corresponding drawings (Chapter 4)- Sagittal series shows the complete mediolateral extent of the left hemisphere in 30 sagittal MRI sections, with 30 corresponding drawings (Chapter 5). The sagittal series also illustrates the location of different fiber tracts in the white matter- Individual variability - provides selected cortical and subcortical areas in three-dimensional MRI (horizontal, coronal, and sagittal MRI planes). For comparison, it also provides similar areas in coronal MRI section in six other monkeys. (Chapter 6)- Vasculature - indicates the corresponding location of all major blood vessels in horizontal, coronal, and sagittal series of sections- Provides updated information on the cortical and subcortical areas, such as architectonic areas and nomenclature, with references, in chapter 2- Provides the sterotaxic grid derived from the in-vivo MR image
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Chapter 1
Introduction, Methods and Presentation of Data
Introduction
Materials and Methods
MRI data collection
Histological processing
Immunohistochemical procedures
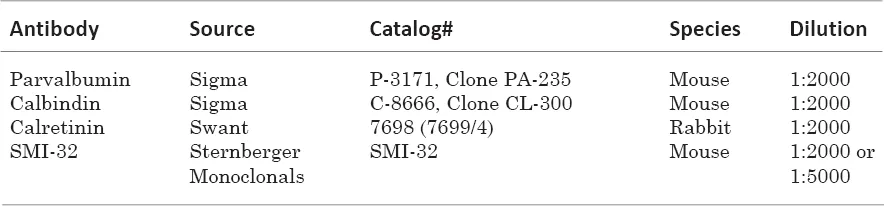
Data analysis
Table of contents
- Cover image
- Title page
- Table of Contents
- Dedication
- Copyright
- Preface
- About the Authors
- Acknowledgments
- Comments
- Chapter 1. Introduction, Methods and Presentation of Data
- Chapter 2. Cytoarchitectonic and Chemoarchitectonic Organization of Cortical and Subcortical areas
- Chapter 3. Horizontal Series
- Chapter 4. Coronal Series
- Chapter 5. Sagittal Series
- Chapter 6. Selected Cortical and Subcortical Areas in three Different MRI Planes and Different Cases
- Index of Abbreviations